Abstract
Objectives
The objective of the study was to evaluate the results of nasolabial/extended nasolabial flaps as a modality for treatment of oral submucous fibrosis.
Materials and Methods
Eleven patients of Stage III or IVa maximum interincisal opening were selected to be operated. Nasolabial/extended nasolabial flaps were done for both the sides. All of the flaps were done in a single stage and were inferiorly based. A similar flap harvest/surgical technique was utilized for all the cases.
Results
The preoperative mouth opening ranged from 5 to 16 mm, with a mean of 10.09 mm. At 6 months the mouth opening ranged from 29 to 39 mm. Some of the complications encountered were poor scar, wisdom tooth traumatising the flap, decreased mouth opening due to non compliance and too much bulk. All of theses were managed satisfactorily.
Oral submucous fibrosis (OSF) is a chronic, progressive, precancerous disease most prevalent in South East Asia and South Asia; its incidence in South East Asia ranges from 0.04% to 24.4%1. This disease is characterized by progressive trismus due to fibrous changes in the lamina propria. Clinically, there is vesicle formation, ulceration, pigmentation, stomatitis, alteration in taste, depapillation of the tongue, a burning sensation in the mouth, difficulty with speech, and progressive limitations in mouth opening. In Pakistan, Karachi has the highest incidence of OSF due to the very frequent use of the areca nut as an ingredient in pan, chalia, ghutka, etc. OSF was first described by Schwartz in 1952. Khanna and Andrade2 have classified OSF based on maximal interincisal opening (MIO): Stage I, early OSF without trismus (MIO >35 mm); Stage II, mild to moderate disease (MIO 26–35 mm); Stage III, moderate to severe disease (MIO 15–25 mm); Stage IVa, severe disease (MIO <15 mm); and Stage IVb, extremely severe malignant/premalignant lesions noted intraorally. OSF can be managed medically and surgically. Medical management is reserved for Stages I and II and consists of intralesional injections of hyaluronidase, steroids and placental extracts345, vitamins, and iron supplements67. Submucosal injections of some drugs have been attempted but have led to increased morbidity, aggravation of trismus, and fibrosis from the mechanical trauma of needle insertion89. Surgical options include excision of fibrous bands with knife cautery or laser, followed by reconstruction of the defect with grafts. Attempts at surgical excision of the bands as a single procedure have failed. Reconstructive options include local pedicled flaps, such as skin and tongue flaps10, greater palatine artery flaps2, buccal fat pads5, nasolabial flaps8, temporalis fascia flaps11, and free vascularized flaps, such as the radial forearm free flap.
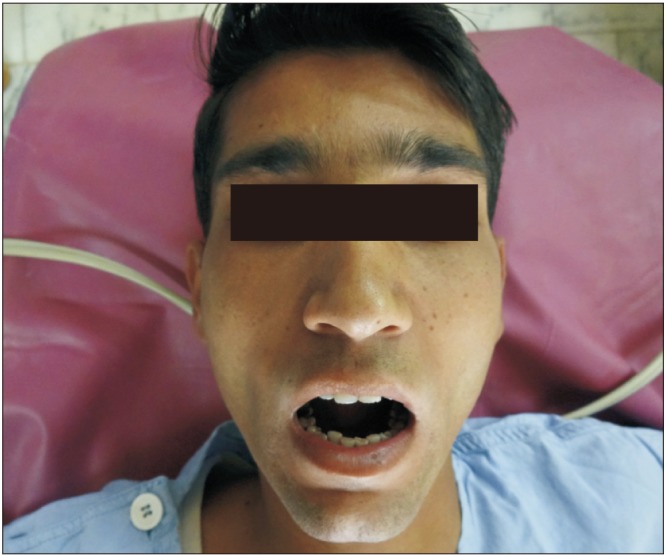
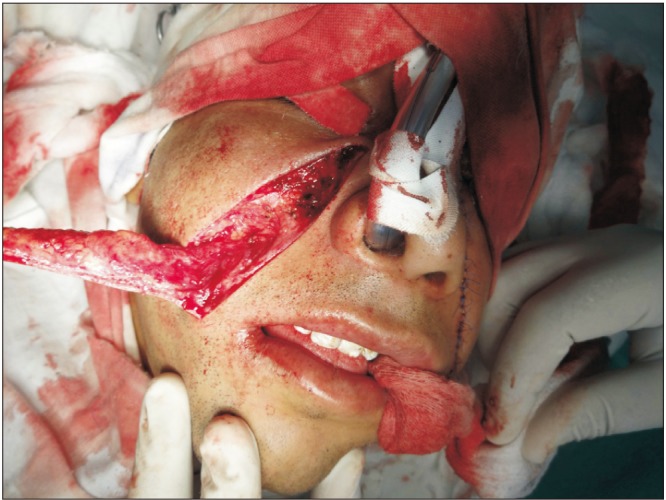
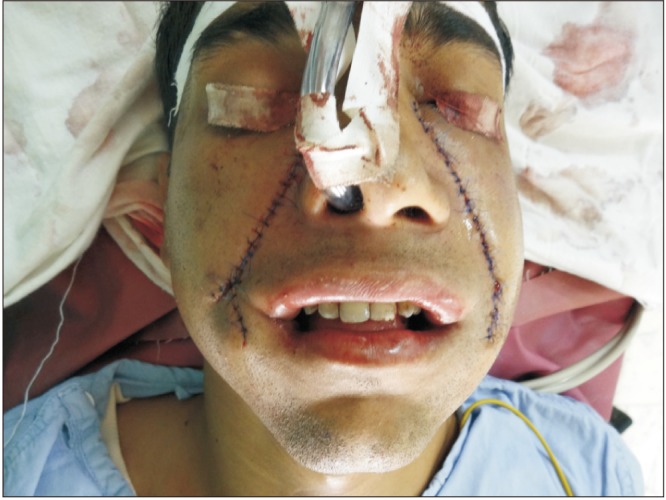
Eleven patients were surgically treated for OSF by all authors. Both surgeons followed the same protocol steps in all procedures. All patients were Khanna and Andrade Stage III or IVa. Patients ranged in age from 17 to 29 years and included nine male and two female patients. All patients were otherwise healthy and had no comorbidities. Upon clinical examination, participants presented with variable degrees of decreased mouth opening. The chief complaint was an inability to open the mouth, and one patient also complained of a burning sensation in the mouth and excessive irritation in response to spicy food. Nine patients had received some form of medical treatment to control the disease process, including intralesional injections of corticosteroids and hyaluronidase and oral forms of pentoxyfylline and colchicine. Two patients refused any attempt at medical treatment and requested surgical intervention instead. Iron supplementation was given to two patients to correct iron-deficiency anemia. The surgeries were carried out under general anesthesia with fiber optic nasal intubation. Preoperative consent for emergent tracheostomy was obtained if required in cases of failure of fiber optic nasal intubation; however, this procedure was not required in any of our cases. Preoperatively, 1.2 g of coamoxiclav was injected into every patient as a prophylactic antibiotic. Fibrous band excision was carried out intraorally with an electrosurgical knife, from the oral commissure (without extending extraorally) to the retromolar region. Judicious undermining of the wound margins was ensured to allow release of trismus and also to facilitate closure. If the mouth opening achieved was less than 35 mm after bilateral fibrous band excision, then intraoral bilateral coronoidectomy with release of the temporalis muscle fibers was completed through the same incision. In all cases, the intraoperative mouth opening achieved was equal to or greater than 35 mm. A Fergussen mouth gag was used to open the mouth and record the MIO.(Fig. 1, 2) The nasolabial flap was harvested laterally from the oral commissure to just inferomedial to the medical canthus. The flap width was approximately 1.5 to 2 cm at the base and gradually tapered to around 0.25 to 0.5 cm superiorly.(Fig. 3) The medial limb of the incision was intentionally left longer than the lateral limb of the incision. The flap was raised in a supramuscular plane from superior to inferior with blunt dissection. The pedicle was positioned at the approximate location of the modiolus, and a transbuccal tunnel was created medial to it. This transbuccal tunnel was widened to the width of two fingers so that the flap could be transferred intraorally without any tension on the pedicle. The buried/in turned portion of the flap was de-epithelialised and then sutured with 4-0 Vicryl. The donor site was closed in layers with 4-0 Vicryl and 5-0 Prolene, taking care not to damage the pedicle.(Fig. 4) Wound cleansing with chlorhexidine mouthwash was carried out four times a day for the first three weeks postoperatively. Mouth opening exercises were started from the seventh postoperative day using wooden sticks at a frequency of three times a day. Beginning on the 15th postoperative day, the frequency and duration of these mouth opening exercises were increased to attain the bare minimum target of a 35-mm interincisal opening.(Fig. 5, 6, 7, 8) One of our patients had a relapse when he did not return for many weeks of follow-up. He was subsequently given intravenous sedation, and mouth opening of 35 mm was achieved. The patients were given multivitamin tablets to be taken daily for six months and were motivated regarding permanent discontinuation of any habits that had influenced their condition. They were also advised to see a dietician and begin a healthy diet rich in fruits and vegetables as part of a healthy lifestyle.
There were a total of 11 patients in our series: 9 males and 2 females. The age of the patients ranged from 17 to 29 years, with a mean age of 23.36 years. Of 11 patients, 6 patients had a habit of betel nut chewing, and the other five used quid and snuff. The length of history of exposure to these agents ranged from 2 to 19 years, with a mean exposure time of 7.72 years. Of 11 patients, 3 patients (27.3%) were classified as Khanna and Andrade Stage III, and the remaining 8 patients (72.7%) were classified as having Stage IVa disease. The preoperative mouth opening ranged from 5 to 16 mm, with a mean of 10.09 mm. Six months after the surgical intervention and aggressive physiotherapy exercises, the average postoperative mouth opening ranged from 29 to 39 mm, with a mean of 32.9 mm. Student's t-test showed statistically significant improvement in mouth opening with nasolabial flap (P>0.05). Eight patients underwent a simple nasolabial flap, while the remaining three had advanced disease extending up to the commissure and therefore required an extended nasolabial flap for complete coverage of their defect. One patient had asthma, and two others had anemia due to an inability to take enough food. One female patient required scar revision to improve her facial esthetics. In another patient, debulking of the flap was needed, while another case underwent extraction of a wisdom tooth to prevent flap trauma. Only one patient presented with a mild dehiscence of the flap in the retromolar area, and this flap was salvaged by resuturing. The results are shown in Table 1.
OSF is a progressive and potentially debilitating condition in which there is interference with regular inspection of the oral cavity and compromises in food intake and speech12. Habitual chewers of betel nuts experience increased muscle activity that results in progressive degeneration, scarring, and fibrosis of the muscle13. There are generally two options to treat OSF: medical and/or surgical. Cases in Stage I and Stage II (Khanna and Andrade) are treated medically, while Stages III and IV are managed surgically. All patients in our study were offered medical treatment for symptom control; two patients refused any form of medical treatment and were managed surgically, while medical treatment failed to produce the desired result in the remaining nine cases due to the advanced nature of the disease. Surgical release of fibrous bands without vascularized tissue coverage does not produce good results. Split thickness grafting after fibrous band release has a high recurrence rate due to graft shrinkage. Other options include palatal island flaps, bilateral tongue flaps3, buccal fat pads, bilateral forearm free flaps14, temporalis pedicled flaps11, superficial temporal fascia flaps11, anterolateral thigh flaps15, collagen sheets3, placental grafts16, and allografts17. Our choice of flap was a nasolabial flap and depended upon the length of the flap required and the severity of the fibrosis involving the commissure. We used either an inferiorly based nasolabial flap or a centrally based nasolabial flap/extended nasolabial flap. Nasolabial flap is a very dependable axial pattern flap with a mobile pedicle that can be safely transposed intraorally. Because it has a longer and more versatile pedicle, the flap can be extended to the area of oral commissure18. We created a total of 22 nasolabial flaps for 11 patients. None of the patients had any significant complications with regard to flap failure. We also completed scar revision for extra-oral scarring in one patient, while another required extraction of a wisdom tooth to prevent trauma to the flap. In one case, flap debulking and revision were performed. One patient presented with an approximately 1-cm dehisced segment in the retromolar region, which was resutured. Nasolabial flaps have many advantages, including a reliable blood supply, ease of elevation, minimal esthetic deformity, supple skin, and proximity to the defect site. Some of the disadvantages are intraoral hair growth and scarring in some patients, especially young females. All patients were pleased with the postoperative mouth opening. Only one patient reported decreased mouth opening, which was due to noncompliance with the recommended physiotherapy following surgery. There was no case of flap dehiscence, infection, or damage to Stenson's duct or any branches of facial nerves. All patients were given postoperative mouth opening exercises with the aim of maintaining a minimum postoperative mouth opening of 35 mm. Although this was not achievable in every case, a mouth opening of at least 30 mm was achieved in all patients except two, who were at 29 mm. All patients were also referred for hair removal with laser treatment. Only one patient visited a dermatologist for permanent hair removal. The patients were satisfied with the mouth opening and the extraoral scarring. The importance of regular follow up and physiotherapy was emphasized to the patients on every visit. The follow-up period in our series ranged from 7 to 40 months. The follow-up regime consisted of thrice weekly visits for the first two weeks, twice weekly for the next two weeks, and then once a week for three months. After that, the patients were asked to visit once a month for the next six months and then every six months for the next three years.
OSF is a chronic, debilitating disease in which there is a decrease in mouth opening that can only be reversed through surgical excision of fibrous bands, followed by reconstruction of the defect by transferring tissue from a local or a distant site. There are many reconstructive options, and each has its own pros and cons. We used either an inferiorly based nasolabial flap or a centrally based nasolabial flap (if the fibrosis extended to the oral commissure). The cornerstone of a successful postoperative result lies in habit cessation, lifestyle changes, and aggressive physiotherapy.
Notes
Authors' Contributions: M.U.Q. and O.S.J. were the chief operating surgeons. E.U.H. and R.Z. assisted them in operations and helped them in study design and manuscript writing.
References
1. Pindborg JJ, Murti PR, Bhonsle RB, Gupta PC, Daftary DK, Mehta FS. Oral submucous fibrosis as a precancerous condition. Scand J Dent Res. 1984; 92:224–229. PMID: 6589738.

2. Khanna JN, Andrade NN. Oral submucous fibrosis: a new concept in surgical management. Report of 100 cases. Int J Oral Maxillofac Surg. 1995; 24:433–439. PMID: 8636640.
3. Mehrotra D, Pradhan R, Gupta S. Retrospective comparison of surgical treatment modalities in 100 patients with oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:e1–e10.

4. Gupta D, Sharma SC. Oral submucous fibrosis--a new treatment regimen. J Oral Maxillofac Surg. 1988; 46:830–833. PMID: 3171741.

5. Yeh CJ. Application of the buccal fat pad to the surgical treatment of oral submucous fibrosis. Int J Oral Maxillofac Surg. 1996; 25:130–133. PMID: 8727586.

6. Aziz SR. Oral submucous fibrosis: case report and review of diagnosis and treatment. J Oral Maxillofac Surg. 2008; 66:2386–2389. PMID: 18940512.

7. Bhishagratna KKL. Chapter XVI. Diagnosis of disease of mouth. In : Bhishagratna KKL, editor. An English translation of the Susruta Samhita: Nidána-Sthána to Kalapa-Sthána. Vol 2. Calcutta: Selbstverl;1911. p. 111–112.
8. Borle RM, Nimonkar PV, Rajan R. Extended nasolabial flaps in the management of oral submucous fibrosis. Br J Oral Maxillofac Surg. 2009; 47:382–385. PMID: 18995935.

9. Jiang X, Hu J. Drug treatment of oral submucous fibrosis: a review of the literature. J Oral Maxillofac Surg. 2009; 67:1510–1515. PMID: 19531426.

10. Huang IY, Wu CF, Shen YS, Yang CF, Shieh TY, Hsu HJ, et al. Importance of patient's cooperation in surgical treatment for oral submucous fibrosis. J Oral Maxillofac Surg. 2008; 66:699–703. PMID: 18355593.

11. Mokal NJ, Raje RS, Ranade SV, Prasad JS, Thatte RL. Release of oral submucous fibrosis and reconstruction using superficial temporal fascia flap and split skin graft--a new technique. Br J Plast Surg. 2005; 58:1055–1060. PMID: 16055096.

12. Chao CK, Chang LC, Liu SY, Wang JJ. Histologic examination of pedicled buccal fat pad graft in oral submucous fibrosis. J Oral Maxillofac Surg. 2002; 60:1131–1134. PMID: 12378485.

13. Chang YM, Tsai CY, Kildal M, Wei FC. Importance of coronoidotomy and masticatory muscle myotomy in surgical release of trismus caused by submucous fibrosis. Plast Reconstr Surg. 2004; 113:1949–1954. PMID: 15253182.

14. Lee JT, Cheng LF, Chen PR, Wang CH, Hsu H, Chien SH, et al. Bipaddled radial forearm flap for the reconstruction of bilateral buccal defects in oral submucous fibrosis. Int J Oral Maxillofac Surg. 2007; 36:615–619. PMID: 17499479.

15. Huang JJ, Wallace C, Lin JY, Tsao CK, Kao HK, Huang WC, et al. Two small flaps from one anterolateral thigh donor site for bilateral buccal mucosa reconstruction after release of submucous fibrosis and/or contracture. J Plast Reconstr Aesthet Surg. 2010; 63:440–445. PMID: 19359228.

16. Lai DR, Chen HR, Lin LM, Huang YL, Tsai CC. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10-year experience with 150 cases. J Oral Pathol Med. 1995; 24:402–406. PMID: 8537913.

17. Ko EC, Shen YH, Yang CF, Huang IY, Shieh TY, Chen CM. Artificial dermis as the substitute for split-thickness skin graft in the treatment of oral submucous fibrosis. J Craniofac Surg. 2009; 20:443–445. PMID: 19276824.

18. Gewirtz HS, Eilber FR, Zarem HA. Use of nasolabial flap for reconstruction of the floor of the mouth. Am J Surg. 1978; 136:508–511. PMID: 360855.
Table 1
Description of NLFs used in oral submucous fibrosis
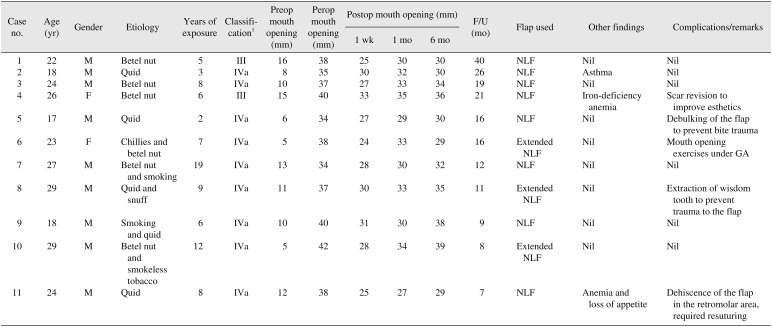
(NLFs: nasolabial flaps, M: male, F: female, Preop: preoperative, Perop: peroperative, Postop: postoperative, Nil: none, GA: general anesthesia)
1Khana and Andrade's classification: Stage III, moderate to severe disease (maximal interincisal opening [MIO] 15–25 mm); Stage IVa, severe disease (MIO <15 mm).




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download