Abstract
Objectives
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy worldwide. Inconsistency in various histopathologic features for predicting nodal metastasis and overall prognosis and a better understanding of molecular mechanisms of tumourigenesis have shifted the focus to a search for more definitive predictive markers. To identify the role of two immunohistochemical (IHC) markers, E-cadherin and cyclin D1, as predictive markers of aggressiveness in HNSCC and to assess clonal expansion of tumour cells.
Materials and Methods
A total of 66 cases of HNSCC with neck node dissection were studied. IHC was performed on primary tumour sections and lymph nodes showing metastatic deposits. Histopathological parameters such as tumour grade and TNM stage together with nodal status were compared according to expression of the two markers. Fischer's chi-square test was used to assess the correlation between the two markers and histopathological parameters.
Results
Out of 66 cases studied, 37 showed LN metastasis. Most of the patients were male, and the most common tumour site was buccal mucosa. We found a significant association between loss of E-cadherin and node metastasis (P<0.001) and higher TNM stage (P<0.001). Cyclin D1 overexpression was significantly associated with only nodal metastasis (P=0.007). No significant association with tumour grade was found for either marker. The subgroup of E-cadherin loss with cyclin D1 overexpression was associated with the maximum incidence of nodal metastasis and higher TNM stage, highlighting the importance of using a combination of these two markers. A significant association was noted between the expression of markers at the primary site and at nodal deposits, indicating clonal expansion.
Head and neck squamous cell carcinoma (HNSCC) ranks sixth among the most common neoplasms in the world and is responsible for more than 350,000 cancer deaths worldwide every year,123. In India, where tobacco is the most common etiologic factor, HNSCC is the most common cancer in men and the third most common in women, with an increasing incidence reported in young adults1.
Despite an aggressive multidisciplinary approach and advances in treatment, the 5-year survival rate for HNSCC has not improved much4. Although a number of factors such as TNM stage56, histologic grade of tumour78, perineural invasion89, depth of tumour8, and extracapsular spread1011 have been used as prognostic markers in HNSCC, regional lymph node (LN) metastasis has been considered one of the most important factors determining treatment approach and thus long-term survival8101112. Even with advances in diagnostic imaging the sensitivity to detect occult LN metastasis remains limited, especially in Stages I and II1314. Furthermore, it has been observed that even among patients with same stage and site of HNSCC, some patients do better than others1. The past two decades saw innovative research in a number of molecular studies on the identification of occult LN metastasis. These studies have shown that development of HNSCC is driven by multistep accumulation of genetic alterations involving various oncogenes, tumour suppressor genes, and cell adhesion molecules, resulting in a heterogeneous cell population with probable differences in invasive and metastatic behaviour and clonal proliferation of these cells11314151617. This has led to increased interest in assessing cancer progression in HNSCC and predicting its course using different molecular markers, which has in turn fuelled the search for markers that can act as definitive prognostic/predictive markers. Furthermore, tumour heterogeneity has led to the use of multiple selected markers together with histopathology in prognostic assessment of HNSCC151819.
E-cadherin is a 120-kDa calcium-dependent transmembrane glycoprotein encoded by the CDH1 tumour suppressor gene located on chromosome 16q21. It is a cell adhesion molecule that connects epithelial cells via homotypic interaction. Disruption of this interaction promotes detachment of cancer cells from their primary sites, the first step in the tumour invasion process4. Reduced or aberrant E-cadherin expression appears to be associated with various parameters such as aggressiveness of tumour, enhanced invasion, and metastatic potential in a number of malignancies including HNSCC20212223. The cyclin D1 gene (CCND1) located on chromosome 11q13 encodes a nuclear protein that is the regulatory subunit of Cdk-4 and Cdk-6. Cyclin D1 plays a major role in cell cycle transition from G1 to S phase by contributing to inactivation of the retinoblastoma (RB) gene product, and overexpression of CCND1 has been reported in 35% to 40% cases of HNSCC2425.
We conducted a study to assess the loss of E-cadherin (tumour suppressor gene) and overexpression of cyclin D1 (proto-oncogene) in primary tumours and metastatic nodes by immunohistochemistry (IHC) to evaluate their role as predictive markers of nodal metastasis and assess clonal expansion in HNSCC patients at our tertiary care centre.
We selected 66 patients with HNSCC who had undergone wide local excision of primary tumour with simultaneous selective resection of regional LNs between January 2015 and December 2016, and for whom complete clinical data and surgical pathology material were available for review and IHC. HNSCC patients who underwent neoadjuvant therapies at presentation and those with distant metastasis were excluded from the study. Pathology reports and paraffin-embedded tissue blocks were retrieved from the archives and data on demographics and clinical profile including age, sex, primary tumour site, TNM stage, and histopathologic grade were collected. H&E sections of representative primary tumour were reviewed by two independent pathologists for confirmation of diagnosis and graded according to Broder's histologic criteria for HNSCC. TNM staging (Stage I-IVA) was performed according to American Joint Committee on Cancer (AJCC) 7th edition.
The study was approved by Institutional Ethics Committee of Armed Forces Medical College, Pune, India (approval no. pathology and allied/32/2015-17).
Blocks of primary site tumour covering at least 60% of the total section (in N0 cases) together with sections of the LN showing largest metastatic deposits (in case of N1-2) were selected. Sections of 3 to 4 µm thickness were cut onto coated slides for IHC using mouse monoclonal antibody against E-cadherin (clone 36 [Biogenix, Memphis, TN, USA]; ready to use, incubation period 30 minutes) or rabbit polyclonal antibody against cyclin D1 (clone EP12 [PathnSitu Biotechnologies, Livermore, CA, USA]; ready to use, incubation period 30 minutes). IHC was performed by the standard Avidin-Biotin technique according to the manufacturer's instructions. A section of normal mucosa and a section of tonsil were taken as positive controls for E-cadherin and cyclin D1 respectively. For negative controls, primary antibody was omitted and Tris-buffered saline was used for both markers.
Intensity of membranous staining for E-cadherin26 and percentage of cells showing nuclear positivity for cyclin D127 were evaluated semi-quantitatively by two independent pathologists.(Table 1, Fig. 1, 2) All cases were classified in a binary fashion as positive or negative for each marker using a pre-decided scoring system.(Table 1) Cytoplasmic staining was disregarded for both markers.
The IBM SPSS Statistics (ver. 20.0; IBM Co., Armonk, NY, USA) was used for statistical analysis. Association with E-cadherin and cyclin D1 was analysed for different clinicopathologic parameters. The chi-square test and the Fisher's exact probability test were used for comparisons of categorical data. The statistical significance was determined at P<0.05 for all values.
A total of 66 cases of HNSCC were included in the study, of which 37 cases (56.1%) had metastatic LNs and 29 cases (43.9%) were without nodal metastasis. The mean age of the study group was 57 years, with a standard deviation of 13.1 years. Most cases were in the age group of 41 to 60 (33 cases, 50.0%) and males outnumbered females. The major subsite was buccal mucosa (33 cases, 50.0%) and only one case (1.5%) involved the hard palate. Histologically, 43 cases (65.2%) were well-differentiated (low grade) and 23 cases (34.8%) were moderately differentiated or poorly differentiated HNSCC (classified as high grade). All cases were binarily classified into early (19 cases, 28.8%) or advanced stage (47 cases, 71.2%) based on TNM staging.(Table 2)
Expression of E-cadherin and cyclin D1 by IHC was analysed at the primary tumour site and the metastatic nodal deposit. All cases (n=66) retained expression of E-cadherin at the primary tumour site and membranous immunostaining was seen in 34 cases (51.5%), whereas E-cadherin loss was noted in 21 of 37 metastatic LNs (56.8%). Similarly, overexpression of cyclin D1 was observed in the form of nuclear positivity at the primary site tumour in 32 cases (48.5%) and in 19 of 37 metastatic nodal deposits (51.4%).(Table 3)
A highly significant association was observed between E-cadherin expression and LN status in HNSCC (P<0.001). A significant association was also noted between E-cadherin expression and TNM stage (P<0.001), indicating that loss of E-cadherin expression is associated with higher TNM stage and LN metastasis. However, there was no significant association with histologic tumour grade (P=0.103). In addition, a significant statistical association (P=0.007) was noted between expression of E-cadherin in primary tumour and expression in metastatic LN in node-positive cases (n=37).(Tables 4, 5)
A significant association was present between LN status and cyclin D1 expression (P=0.044) suggesting an increased rate of LN metastasis in cases with overexpression of cyclin D1. However, no significant association was noted between TNM stage and cyclin D1 expression (P=0.081) or between tumour grade and cyclin D1 expression (P=0.266). Similar to E-cadherin, there was a highly significant association (P<0.001) between expression of cyclin D1 in primary tumour and expression in metastatic LN deposits in nodepositive cases (n=37).(Tables 4, 5)
All 66 cases were further divided into subgroups based on combined expression of E-cadherin and cyclin D1. On subgroup analysis of 37 cases with LN metastasis, the highest rate of LN metastasis was seen in the subgroup of reduced E-cadherin/cyclin D1 overexpression (negative/positive) with a rate of 43.2% (16 cases), while the lowest rate (2 cases, 5.4%) was observed in the subgroup of retained E-cadherin/low cyclin D1 expression. In contrast, among 29 cases without LN metastasis, only one (3.4%) case was in the subcategory of E-cadherin loss/cyclin D1 overexpression (negative/positive) whereas most cases (58.6%) were in the subgroup of retained E-cadherin/absent cyclin D1 (positive/negative). Thus, a significant statistical association was observed between LN metastasis and the combination of E-cadherin and cyclin D1 expression in tumour cells (P=0.001).(Table 6) Similarly, a significant association was noted with TNM stage (P=0.001), with advanced TNM stages mostly seen in the subgroup of low E-cadherin/overexpressed cyclin D1 (17/47 cases, 36.2%).(Table 6)
Traditionally, various histopathologic features such as depth of tumour8, pattern of invasion78, tumour differentiation, and TNM stage56 have been used as predictive and prognostic markers in HNSCC. However, the lack of accurate imaging modalities to detect occult or micro-metastasis, combined with the fact that tumours showing the same TNM stage and location behave differently and a lack of consensus on management of draining LNs in TNM stage I and II, has raised serious doubts regarding the adequacy of conventional markers1314. In the past two decades, extensive research has been carried out involving various biomarkers such as p53, beta-catenin, E-cadherin, and cyclin D1 to search for a definitive predictive and prognostic biomarker in HNSCC1819. We studied the importance of E-cadherin and cyclin D1 as combination markers in terms of their relationship to clinicopathologic parameters to examine their role as predictive markers in HNSCC and also examined their expression in metastatic LNs for assessment of clonal proliferation.
The mean age of the study group was 57 years with a standard deviation of 13.1 years, which is similar to the study of Do et al.28 in which the mean age of patients was 61.6 years with no case under 25 years of age and the male:female ratio was 130:16. Loss of E-cadherin expression in primary site tumour (irrespective of comparison of nodal status) was seen in 48.48% cases and cyclin D1 overexpression was observed in 48.45% cases, compared with rates of 77% and 69%, respectively, reported by Do et al.28 and 50.7% and 29.3% reported by Ahmed et al.29. Furthermore, a review of literature revealed great variance in values, ranging from 29.3% to 69%. This variation might be due to different scoring systems used in evaluations, such as the cutoff for cyclin D1 overexpression ranging from 5% to 50% cells. The choice of cutoff values for various markers in HNSCC to distinguish positive from negative results usually lacks a fundamental basis and is rather arbitrary30.
In our study, 29 cases (78.4%) with nodal metastasis showed loss of E-cadherin expression while 26 cases (89.7%) without metastatic nodes showed retained E-cadherin expression (Table 4); this indicates that E-cadherin loss is significantly higher in cases with LN metastasis and is in agreement with various other studies2829303132. Akhtar et al.33 and Kaur et al.34 reported loss of E-cadherin in 5/6 and 7/10 cases (respectively), including cases with LN metastasis. Their results differ from those of Balasundaram et al.35, Wang et al.36, Mattijssen et al.37, and Mehendiratta et al.1, who reported prognostic significance of E-cadherin in HNSCC cases. Regarding tumour differentiation, which is considered to be an independent prognostic marker, we did not find any significant association between tumour grade and E-cadherin expression in our study, which is in concordance with results of several past studies28303839 but differs from some other studies293334. This discrepancy may be due to subjective variation in grading the tumour, a limitation of Broder's system of grading HNSCCs, as a number of stage IV tumours in our study were graded as well differentiated. Also, loss of E-cadherin was significantly higher in advanced TNM stages III and IVA, similar to a report by Ahmed et al.29 involving only laryngeal SCCs. In our study, 94.7% of early-stage cases showed normal expression of E-cadherin whereas only 34% of advanced-stage cases retained E-cadherin expression. However, this finding differs from most previous studies, which have not reported any significant association between E-cadherin expression and TNM stage283035.
Cyclin D1 overexpression was also found to be significantly associated with LN metastasis.(Table 4) These results are comparable to a number of previous studies including those of Do et al.28, Ahmed et al.29, Carlos de Vicente et al.40 and a few Indian studies314142, but in discordance with studies by Michalides et al.43 and Takes et al.30. Regarding TNM stage, few studies in the literature have reported a significant association between TNM stage and cyclin D1 expression282944. In our study we did not find a statistically significant association (P=0.081) although we noted that 68.4% of early-stage tumours did not show cyclin D1 overexpression. No significant association was noted with tumour grade, which is in agreement with previous studies2842, although there are a few reports of a significant correlation between tumour grade and cyclin D1 overexpression2941444546.
Furthermore, among 37 cases with nodal metastasis, 29 showed loss of E-cadherin at the primary tumour site of which 21 cases (72.4%) also had loss of E-cadherin at secondary nodal deposits.(Table 5) This statistically significant association (P=0.007) indicates that the sub-clone of tumour cells that had lost E-cadherin expression proliferated and metastasized to the draining LNs. This is the first report of this finding in HNSCCs. However, 8 cases (27.6%) were negative at the primary site but showed positive E-cadherin expression in the LN deposit. In addition, out of 8 cases with LN metastasis that retained E-cadherin expression at primary site, 7 cases (87.5%) retained E-cadherin expression in the nodal deposit and one case (12.5%) showed loss at the secondary site. These cases point toward heterogeneity of the tumour cells, possibly reflecting additional mutations that play a role in carcinogenesis. Also, a highly significant association (P<0.001) was noted between cyclin D1 overexpression at the primary site tumour and at secondary metastatic deposits in node-positive cases, again pointing to clonal expansion. Similar results suggesting tumour heterogeneity were noted with cyclin D1 expression.(Table 5)
The subgroup of cases with loss of E-cadherin/overexpression of cyclin D1 (negative/positive) showed the highest rate of LN metastasis and higher TNM stages (P<0.001).(Table 6) However, 37.9% of the LN-negative group and 51.4% of the LN-positive group showed both markers as either negative or positive, signifying interplay between additional mutations and these markers.
These results imply that overexpression of cyclin D1 as a cell proliferation marker together with loss of the cell adhesion molecule E-cadherin plays a significant role in metastasis with each marker probably influencing the genesis of mutations in other genes. The combination of these markers might help to predict aggressive disease and prognosis in HNSCC.
E-cadherin and cyclin D1 are potential predictive markers of LN metastasis. The combination of these two markers might help identify early-stage HNSCCs at higher risk of LN metastasis, although tumour heterogeneity may interfere with the association in some cases. With emerging evidence of definite roles of different biomarkers in various malignancies, more randomized trials and meta-analysis studies are required to identify the optimal prognostic and predictive markers for HNSCCs.
Notes
Authors' Contributions: K.S. participated in data collection and wrote the manuscript. A.S. supervised the study design and performed the statistical analysis. H.J.P. participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
1. Mehendiratta M, Solomon MC, Boaz K, Guddattu V, Mohindra A. Clinico-pathological correlation of E-cadherin expression at the invasive tumor front of Indian oral squamous cell carcinomas: an immunohistochemical study. J Oral Maxillofac Pathol. 2014; 18:217–222. PMID: 25328302.

2. Iglesias-Bartolome R, Martin D, Gutkind JS. Exploiting the head and neck cancer oncogenome: widespread PI3K-mTOR pathway alterations and novel molecular targets. Cancer Discov. 2013; 3:722–725. PMID: 23847349.

3. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001; 94:153–156. PMID: 11668491.

4. Xu Y, Fisher GJ. Role of met axis in head and neck cancer. Cancers (Basel). 2013; 5:1601–1618. PMID: 24287743.

5. Eiband JD, Elias EG, Suter CM, Gray WC, Didolkar MS. Prognostic factors in squamous cell carcinoma of the larynx. Am J Surg. 1989; 158:314–317. PMID: 2802033.

6. Tytor M, Olofsson J. Prognostic factors in oral cavity carcinomas. Acta Otolaryngol Suppl. 1992; 492:75–78. PMID: 1632258.

7. Stelow EB, Mills SE. Squamous cell carcinoma variants of the upper aerodigestive tract. Am J Clin Pathol. 2005; 124(Suppl):S96–S109. PMID: 16468420.

8. Noguchi M, Kido Y, Kubota H, Kinjo H, Kohama G. Prognostic factors and relative risk for survival in N1-3 oral squamous cell carcinoma: a multivariate analysis using Cox's hazard model. Br J Oral Maxillofac Surg. 1999; 37:433–437. PMID: 10687900.

9. Rahima B, Shingaki S, Nagata M, Saito C. Prognostic significance of perineural invasion in oral and oropharyngeal carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97:423–431. PMID: 15088027.

10. Woolgar JA, Rogers SN, Lowe D, Brown JS, Vaughan ED. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol. 2003; 39:130–137. PMID: 12509965.

11. Puri SK, Fan CY, Hanna E. Significance of extracapsular lymph node metastases in patients with head and neck squamous cell carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2003; 11:119–123. PMID: 14515090.

12. Shingaki S, Takada M, Sasai K, Bibi R, Kobayashi T, Nomura T, et al. Impact of lymph node metastasis on the pattern of failure and survival in oral carcinomas. Am J Surg. 2003; 185:278–284. PMID: 12620571.

13. Close LG, Merkel M, Vuitch MF, Reisch J, Schaefer SD. Computed tomographic evaluation of regional lymph node involvement in cancer of the oral cavity and oropharynx. Head Neck. 1989; 11:309–317. PMID: 2753699.

14. van den Brekel MW, Castelijns JA, Stel HV, Golding RP, Meyer CJ, Snow GB. Modern imaging techniques and ultrasound-guided aspiration cytology for the assessment of neck node metastases: a prospective comparative study. Eur Arch Otorhinolaryngol. 1993; 250:11–17. PMID: 8466744.

15. Takes RP, Baatenburg de, Wijffels K, Schuuring E, Litvinov SV, Hermans J, et al. Expression of genetic markers in lymph node metastases compared with their primary tumours in head and neck cancer. J Pathol. 2001; 194:298–302. PMID: 11439361.

16. Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982; 217:998–1003. PMID: 7112116.

17. Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998; 396:643–649. PMID: 9872311.

18. Cortesina G, Martone T. Molecular metastases markers in head and neck squamous cell carcinoma: review of the literature. Acta Otorhinolaryngol Ital. 2006; 26:317–325. PMID: 17633150.
19. Belbin TJ, Singh B, Smith RV, Socci ND, Wreesmann VB, Sanchez-Carbayo M, et al. Molecular profiling of tumor progression in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2005; 131:10–18. PMID: 15655179.

20. Ren X, Wang J, Lin X, Wang X. E-cadherin expression and prognosis of head and neck squamous cell carcinoma: evidence from 19 published investigations. Onco Targets Ther. 2016; 9:2447–2453. PMID: 27217768.

21. Chaw SY, Abdul Majeed A, Dalley AJ, Chan A, Stein S, Farah CS. Epithelial to mesenchymal transition (EMT) biomarkers--E-cadherin, beta-catenin, APC and vimentin--in oral squamous cell carcinogenesis and transformation. Oral Oncol. 2012; 48:997–1006. PMID: 22704062.
22. Rodrigo JP, Domínguez F, Alvarez C, Manrique C, Herrero A, Suárez C. Expression of E-cadherin in squamous cell carcinomas of the supraglottic larynx with correlations to clinicopathological features. Eur J Cancer. 2002; 38:1059–1064. PMID: 12008193.

23. Yokoyama K, Kamata N, Hayashi E, Hoteiya T, Ueda N, Fujimoto R, et al. Reverse correlation of E-cadherin and snail expression in oral squamous cell carcinoma cells in vitro. Oral Oncol. 2001; 37:65–71. PMID: 11120485.

24. Suh Y, Amelio I, Guerrero Urbano T, Tavassoli M. Clinical update on cancer: molecular oncology of head and neck cancer. Cell Death Dis. 2014; 5:e1018. PMID: 24457962.

25. Lin RJ, Lubpairee T, Liu KY, Anderson DW, Durham S, Poh CF. Cyclin D1 overexpression is associated with poor prognosis in oropharyngeal cancer. J Otolaryngol Head Neck Surg. 2013; 42:23. PMID: 23672832.

26. Götte M, Kersting C, Radke I, Kiesel L, Wulfing P. An expression signature of syndecan-1 (CD138), E-cadherin and c-met is associated with factors of angiogenesis and lymphangiogenesis in ductal breast carcinoma in situ. Breast Cancer Res. 2007; 9:R8. PMID: 17244359.

27. Scantlebury JB, Luo J, Thorstad WL, El-Mofty SK, Lewis JS Jr. Cyclin D1-a prognostic marker in oropharyngeal squamous cell carcinoma that is tightly associated with high-risk human papillomavirus status. Hum Pathol. 2013; 44:1672–1680. PMID: 23566410.

28. Do NY, Park SY, Lim SC. The role of E-cadherin/beta-catenin complex and cyclin D1 in head and neck squamous cell carcinoma. Cancer Res Treat. 2004; 36:72–78. PMID: 20396569.
29. Ahmed RA, Shawky Ael-A, Hamed RH. Prognostic significance of cyclin D1 and E-cadherin expression in laryngeal squamous cell carcinoma. Pathol Oncol Res. 2014; 20:625–633. PMID: 24470282.

30. Takes RP, Baatenburg De, Alles MJ, Meeuwis CA, Marres HA, Knegt PP, et al. Markers for nodal metastasis in head and neck squamous cell cancer. Arch Otolaryngol Head Neck Surg. 2002; 128:512–518. PMID: 12003581.

31. Suresh TN, Hemalatha A, Harendra Kumar ML, Azeem Mohiyuddin SM. Evaluation of histomorphological and immunohistochemical parameters as biomarkers of cervical lymph node metastasis in squamous cell carcinoma of oral cavity: a retrospective study. J Oral Maxillofac Pathol. 2015; 19:18–24. PMID: 26097301.

32. Jiang WG. E-cadherin and its associated protein catenins, cancer invasion and metastasis. Br J Surg. 1996; 83:437–446. PMID: 8665230.

33. Akhtar K, Ara A, Siddiqui SA, Sherwani RK. Diagnostic and prognostic significance of E-cadherin and vimentin in oral cancer metastasis. Ann Pathol Lab Med. 2016; 3:A8–A13.
34. Kaur G, Carnelio S, Rao N, Rao L. Expression of E-cadherin in primary oral squamous cell carcinoma and metastatic lymph nodes: an immunohistochemical study. Indian J Dent Res. 2009; 20:71–76. PMID: 19336864.

35. Balasundaram P, Singh MK, Dinda AK, Thakar A, Yadav R. Study of β-catenin, E-cadherin and vimentin in oral squamous cell carcinoma with and without lymph node metastases. Diagn Pathol. 2014; 9:145. PMID: 25047112.

36. Wang MB, Yip HT, Srivatsan ES. Antisense cyclin D1 enhances sensitivity of head and neck cancer cells to cisplatin. Laryngoscope. 2001; 111:982–988. PMID: 11404608.

37. Mattijssen V, Peters HM, Schalkwijk L, Manni JJ, van't Hof-Grootenboer B, de Mulder PH, et al. E-cadherin expression in head and neck squamous-cell carcinoma is associated with clinical outcome. Int J Cancer. 1993; 55:580–585. PMID: 8406985.

38. Bánkfalvi A, Krassort M, Végh A, Felszeghy E, Piffkó J. Deranged expression of the E-cadherin/beta-catenin complex and the epidermal growth factor receptor in the clinical evolution and progression of oral squamous cell carcinomas. J Oral Pathol Med. 2002; 31:450–457. PMID: 12220351.
39. Shinohara M, Hiraki A, Ikebe T, Nakamura S, Kurahara S, Shirasuna K, et al. Immunohistochemical study of desmosomes in oral squamous cell carcinoma: correlation with cytokeratin and E-cadherin staining, and with tumour behaviour. J Pathol. 1998; 184:369–381. PMID: 9664902.

40. Carlos de Vicente J, Herrero-Zapatero A, Fresno MF, López-Arranz JS. Expression of cyclin D1 and Ki-67 in squamous cell carcinoma of the oral cavity: clinicopathological and prognostic significance. Oral Oncol. 2002; 38:301–308. PMID: 11978554.

41. Das SN, Khare P, Singh MK, Sharma SC. Correlation of cyclin D1 expression with aggressive DNA pattern in patients with tobaccorelated intraoral squamous cell carcinoma. Indian J Med Res. 2011; 133:381–386. PMID: 21537090.
42. Dhingra V, Verma J, Misra V, Srivastav S, Hasan F. Evaluation of cyclin D1 expression in head and neck squamous cell carcinoma. J Clin Diagn Res. 2017; 11:EC01–EC04.
43. Michalides RJ, van Veelen NM, Kristel PM, Hart AA, Loftus BM, Hilgers FJ, et al. Overexpression of cyclin D1 indicates a poor prognosis in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1997; 123:497–502. PMID: 9158396.

44. Zhao Y, Yu D, Li H, Nie P, Zhu Y, Liu S, et al. Cyclin D1 overexpression is associated with poor clinicopathological outcome and survival in oral squamous cell carcinoma in Asian populations: insights from a meta-analysis. PLoS One. 2014; 9:e93210. PMID: 24675814.

45. Saawarn S, Astekar M, Saawarn N, Dhakar N, Gomateshwar Sagari S. Cyclin d1 expression and its correlation with histopathological differentiation in oral squamous cell carcinoma. ScientificWorldJournal. 2012; 2012:978327. PMID: 22629227.

46. Patel SB, Manjunatha BS, Shah V, Soni N, Sutariya R. Immunohistochemical evaluation of p63 and cyclin D1 in oral squamous cell carcinoma and leukoplakia. J Korean Assoc Oral Maxillofac Surg. 2017; 43:324–330. PMID: 29142867.

Fig. 1
Scoring of E-cadherin membranous staining in head and neck squamous cell carcinoma. A. ×10 (score, 0). B. ×40 (score, 1+). C. ×10 (score, 2+). D. ×40 (score, 2+). E. ×10 (score, 3+). F. ×40 (score, 3+).
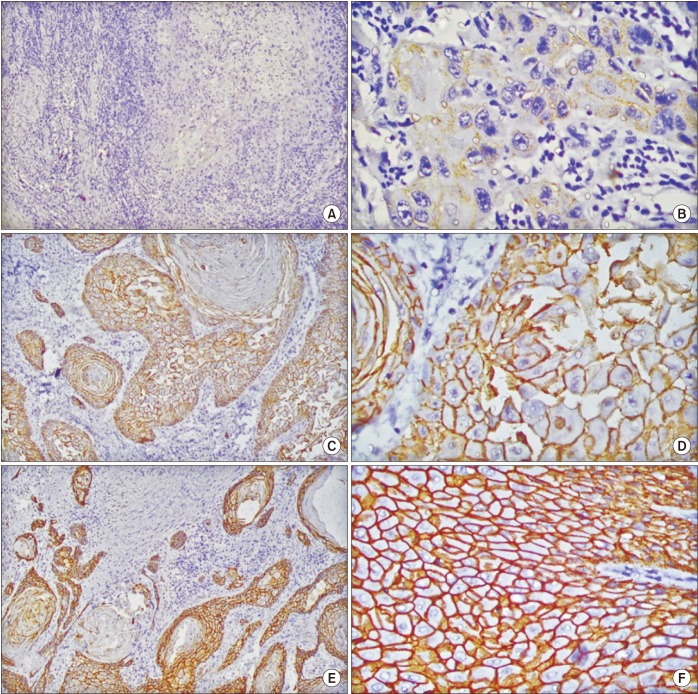
Fig. 2
Scoring of cyclin D1 nuclear staining in head and neck squamous cell carcinoma. A. ×40 (score, 0). B. ×10 (score, 2+). C. ×10 (score, 3+). D. ×40 (score, 3+). E. ×10 (score, 4+). F. ×40 (score, 4+).
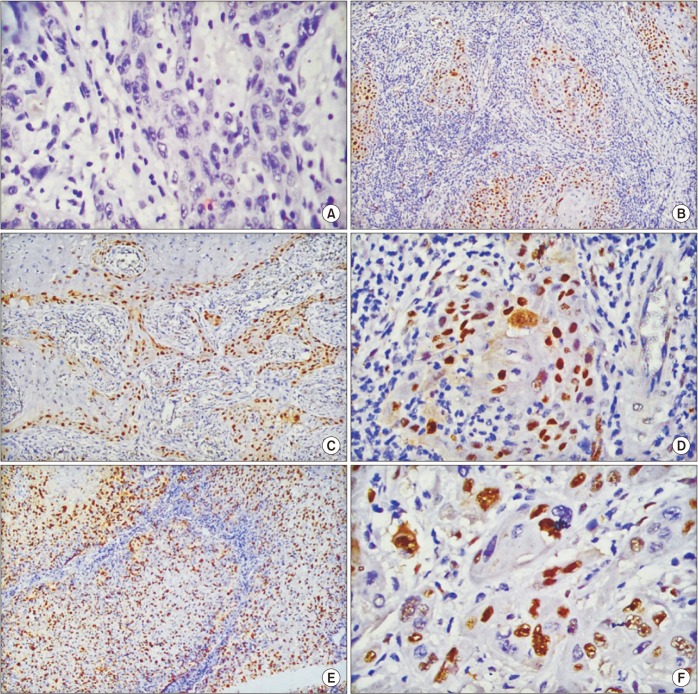
Table 1
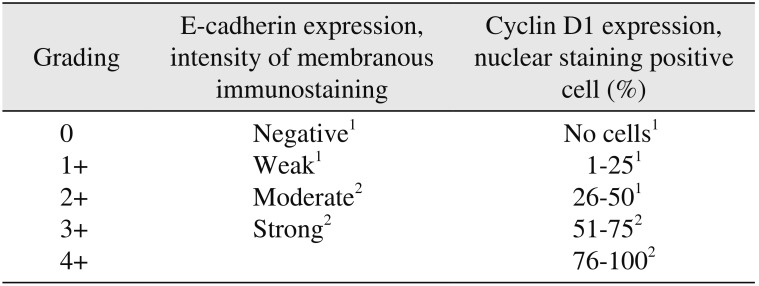
Table 2
Population characteristics of 66 patients with head and neck squamous cell carcinoma
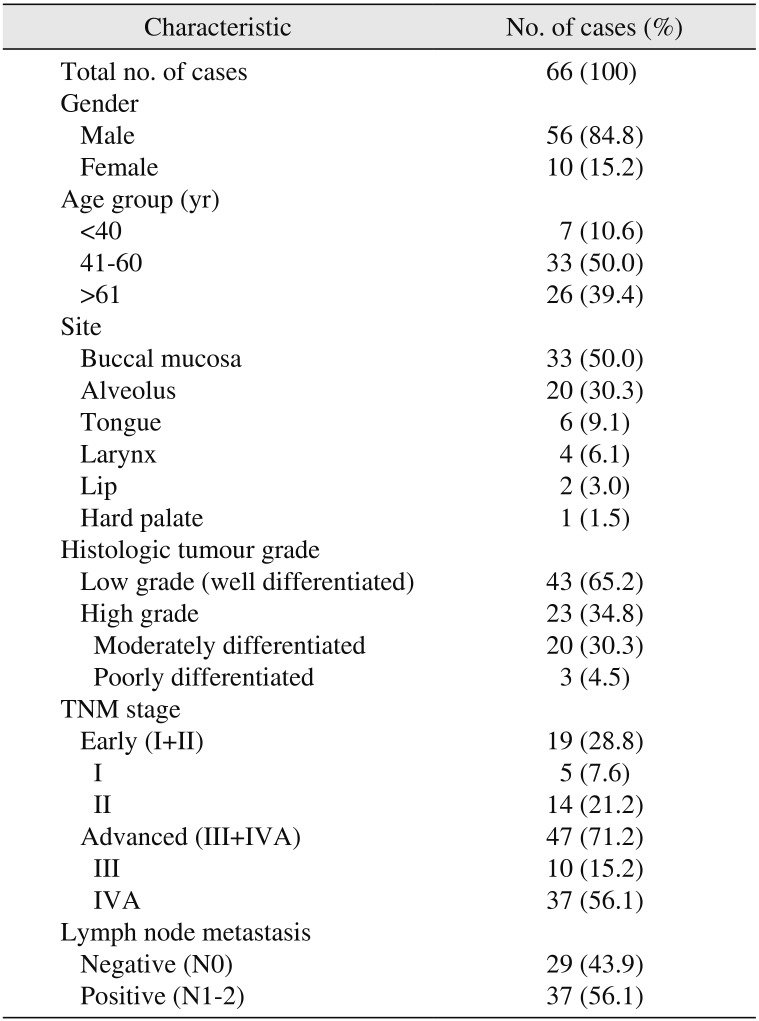
Table 3
Expression of E-cadherin and cyclin D1 by immunohistochemical staining
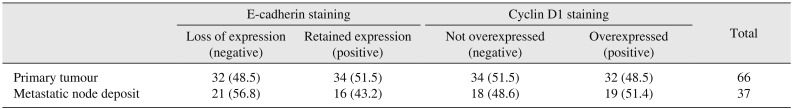
Table 4
Clinicopathological associations with E-cadherin and cyclin D1 in HNSCC
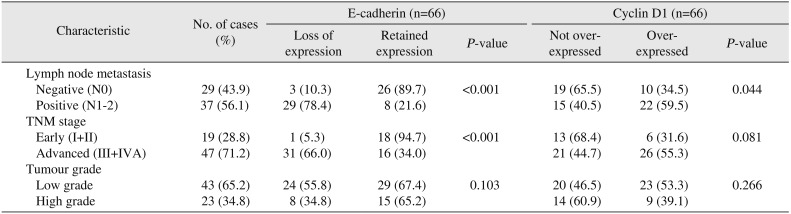
Table 5
Association between expression of E-cadherin and cyclin D1 at primary tumour site and at metastatic nodal deposit in node-positive (N1-2) cases (n=37)
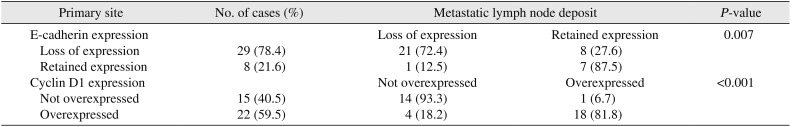
Table 6
Association between E-cadherin and cyclin D1 as combination markers and clinicopathologic parameters (n=66)





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download