Abstract
It has been reported that there are a range of causative drugs related to symmetrical drug-related intertriginous and flexural exanthema (SDRIFE). The causative drugs reported so far include the following: antibiotics, intravenous immunoglobulin, chemotherapeutic agents, and biologics. In this study, we report two cases of SDRIFE and a review of the previous literature. We believe that our study makes a significant contribution to the literature because it demonstrates that intradermal injection of the Chinese herbal ball, and not its topical application, elicited a reaction that predicted the occurrence of SDRIFE. This finding is important for the diagnosis of SDRIFE in future studies. Our findings also provide evidence for a SDRIFE reaction after exposure to ranitidine and mosapride.
Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is a distinctive rash caused by a wide range of drugs. The causative drugs reported so far include antibiotics, intravenous immunoglobulin, chemotherapeutic agents, and biologics1. In this study, we report two cases of SDRIFE.
A 67-year-old male presented with symmetrical erythematous scaly patches on the flexural area (Fig. 1A~E). He had taken Chinese herbal balls composed of poison ivy 3 days prior to the onset of the symptoms. Systemic symptoms were absent, and all laboratory findings were normal. Histopathological findings revealed mild perivascular lymphocytic infiltration. The skin lesions resolved within two weeks, with the use of systemic steroid and antihistamine treatment. Three months later, a patch test for the Chinese herbal ball was applied on his back. The ball was diluted with normal saline at ratios of 1:1, 1:10, 1:100, and 1:1,000. The application sites were observed on D2, D4, and D7, and all findings were negative. Next, we performed a delayed intradermal test on the left forearm. The herbal ball was diluted with normal saline at ratios of 1:10, 1:100, and 1:1,000, and it was injected intradermally. There was a strong positive reaction at the injection site of the 1:10 ratio solution on D2 (Fig. 1F, G).
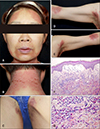
A 50-year-old female patient who exhibited symmetrical erythematous, scaly patches involving the face, neck, inguinal area, and flexural area visited our department (Fig. 2A~E). She had been prescribed ranitidine and mosapride, two days prior to the onset of the aforementioned lesions. Systemic symptoms were absent. All findings of the laboratory tests (complete blood count, eosinophil count, liver function test) were normal. Skin biopsy, in the nape area, revealed dermal edema accompanied by mixed inflammatory cell infiltration (Fig. 2F). There were many eosinophils in the dermis (Fig. 2G). The skin lesions resolved within one week, with the use of systemic steroid and antihistamine treatment. The patient refused to undergo any tests with ranitidine and mosapride.
The diagnostic criteria for SDRIFE include: 1) exposure to a systemically administered drug, either at the first or second administration; 2) sharply demarcated erythema of the gluteal/perianal area and/or V-shaped erythema of the inguinal/perigenital area; 3) involvement of at least one other intertriginous/flexural localization; 4) symmetry of the affected areas; and 5) the absence of systemic symptoms and signs1. In these present cases, all of the features listed above were found. Intradermal skin tests, patch tests, and lymphocyte transformation tests have significant limitations in their utility with respect to the diagnosis1. Barbaud demonstrated that a positive result of patch test to amoxicillin was only found in 50% of patients with SDRIFE2. Results of delayed intradermal tests are not consistent, as negative tests do not correlate with a positive oral provocation test3. The histological characteristics are also nonspecific, and patients exhibit a vast range of symptoms from superficial perivascular inflammatory infiltration, hydropic degeneration of the basal cell layer, to subepidermal bullae and subcorneal pustules3. Although drug provocation tests are still the gold standard for diagnosis, one must exercise a high degree of caution when using these methods1. This is because systemic exposure to the offending drug can elicit a relapse, occasionally presenting with a more serious generalized reaction. In the above-mentioned cases, there is a limitation that oral drugs such as poison ivy can possibly induce a toxic reaction when we perform the intradermal test. We cannot exclude the possibility of a toxic reaction to the 1:10 ratio solution.
It is not known why there is a particular predilection for the flexural areas; however, two hypotheses have been proposed. The first hypothesis is that SDRIFE and baboon syndrome may represent a form of recall phenomenon caused by previous mechanical stimulation or from intertrigo of the flexural areas4. The second probability is that certain drugs or drug metabolites, which receive preference over others, may be excreted from the eccrine gland (the intertriginous and flexural areas are sweat-rich areas5. It is thought that the reaction which occurs from 24 to 48 hours post drug exposure is a type IV hypersensitivity reaction. However, the exanthema appears within hours in a significant number of cases. The direct binding of the drug to T-cell receptors explains the short interval between drug intake and the rash in SDRIFE6. Dermal infiltration by CD3+ and CD4+ T cells has been demonstrated in SDRIFE, accompanied by an expansion of CD26 P-selectin, which generally plays a role in recruitment of type 1 helper T cells to the areas of inflammation7.
We believe that these cases are worth reporting in order to highlight the importance of considering SDRIFE in patients exhibiting a symmetric intertriginous eruption only for a brief period after the intake of drugs. Prompt treatment of this uncommon condition can be achieved through early recognition.
Figures and Tables
Fig. 1
(A~E) Symmetrical erythematous scaly patches on the antecubital fossa, flank, and inguinal area. Delayed intradermal test (F) immediately after injection. (G) Forty-eight hours later, there was a strong positive finding at the injection site of the 1:10 ratio solution. We received the patient's consent form about publishing all photographic materials.
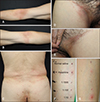
Fig. 2
(A~E) Symmetrical erythematous, scaly patches involving the forehead, perioral area, neck, inguinal area, and flexural area. (F) Dermal edema with mixed inflammatory cell infiltration (H&E, ×100). (G) There were many eosinophils in the dermis (H&E, ×400). We received the patient's consent form about publishing all photographic materials.
ACKNOWLEDGMENT
Author contributions: Dr(s) J Seok, JM Kim, KY Park and SJ Seo had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: J Seok. Acquisition of data: JM Kim. Analysis and interpretation of data: J Seok. Drafting of the manuscript: J Seok, SJ Seo. Critical revision of the manuscript for important intellectual content: KY Park, SJ Seo. Statistical analysis: N/A. Obtained funding: N/A. Administrative, technical, or material support: N/A. Study supervision: KY Park, SJ Seo.
References
1. Tan SC, Tan JW. Symmetrical drug-related intertriginous and flexural exanthema. Curr Opin Allergy Clin Immunol. 2011; 11:313–318.

2. Barbaud A. Skin testing in delayed reactions to drugs. Immunol Allergy Clin North Am. 2009; 29:517–535.

3. Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004; 51:297–310.

4. Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003; 9:2.

5. Valks R, Buezo GF, Daudén E, Fraga J, García-Díez A. Eccrine squamous syringometaplasia in intertriginous areas. Br J Dermatol. 1996; 134:984–986.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download