Abstract
Linear immunoglobulin (Ig) A bullous dermatosis (LABD) is a rare subepidermal autoimmune blistering disease characterized by linear IgA deposits at the basement membrane zone visualized with direct immunofluorescence (DIF). Most cases of LABD are idiopathic, but some are drug-induced with vancomycin being the most common causative agent. We herein report a patient presenting with blisters and erosive lesions, primarily in the intertriginous and flexor areas, consistent with a diagnosis of piperacillin-tazobactam-induced LABD based on the patient's clinical course and histopathology, DIF, and in vitro T-cell activation assay (TAA) findings. Only one case of piperacillin-tazobactam-induced LABD has been previously reported. In addition to its rarity, our case was also unique in that the skin lesions occurred in the intertriginous and flexor areas, uncommon locations for typical adult patients with LABD, and TAA strongly suggested an association with the causative drug.
Linear IgA bullous dermatosis (LABD) is a subepidermal autoimmune bullous disease characterized by linear IgA deposition at the basement membrane zone (BMZ). LABD typically presents with a widespread annular eruption of vesicles and tense bullae1. In adults, the skin lesions usually occur on the trunk, extensor extremities, buttocks, face2, and rarely in the intertriginous and flexor areas3. Involvement of the intertriginous and flexor areas is more commonly seen in children4.
LABD is usually idiopathic, but it may be related to infection or medication5. Multiple drugs have been implicated, and vancomycin is the most common offending medication15. Only one case of piperacillin-tazobactam-induced LABD has been previously reported1. Also, to the best of our knowledge, conventional lymphocyte transformation test (LTT) has been performed in only two previous case reports of drug-induced LABD6. Some recent reports have suggested that T-cell activation assays (TAA) are more sensitive than LTT for the detection of drug-specific responses in patients with a diagnosis of drug hypersensitivity78910. We herein report a patient presenting with blisters and erosive lesions, mainly in the intertriginous and flexor areas. The diagnosis of piperacillin-tazobactam-induced LABD was finally made based on the patient's clinical course and histopathology, direct immunofluorescence (DIF), and TAA findings.
A 59-year-old Taiwanese male was admitted to the hospital with a diagnosis of right lower lung pneumonia. He had a history of insulin-dependent diabetes mellitus, hypertension, end stage renal disease presumed secondary to diabetic nephropathy, peripheral occlusive arterial disease, chronic osteomyelitis of the left foot, and chronic hepatitis C infection. He had no known history of drug allergy and denied recently taking any new medications.
Empirical antibiotic therapy was administered soon after admission with cefuroxime for 1 day. The next day cefuroxime was discontinued and moxifloxacin was administered. Moxifloxacin was discontinued after 3 days, and a 10-day course of piperacillin-tazobactam was begun. In addition, a 7-day course of teicoplanin was added 2 days after the start of piperacillin-tazobactam administration for suspected recurrent osteomyelitis of the left foot. An erythematous skin rash first appeared 3 days after the initial dose of piperacillin-tazobactam, and subsequently the patient developed multiple intense pruritic vesicles, bullae, and erosions primarily on the thighs, axillae, groins, finger webs, and popliteal fossae over the next 7 days. Because of a suspected drug eruption, all antibiotics were discontinued.
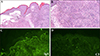
Physical examination revealed numerous clear tense bullae with erosions and crusts on the thighs, axillae, groins, finger webs, and popliteal fossae (Fig. 1A~C). Some lesions were arciform in appearance (Fig. 1D). The ocular, nasal, and genital mucosal membranes were spared. A skin biopsy obtained from an intact blister revealed a subepidermal blister with dense neutrophils, nuclear dust, and eosinophils in the dermis (Fig. 2A, B). DIF demonstrated a continuous linear deposition of IgA and a weak linear deposition of C3 along the BMZ (Fig. 2C, D). Type IV collagen staining showed the subepidermal blister to be above the basement membrane. Indirect immunofluorescence examination failed to demonstrate anti-keratinocyte cell surface or anti-basement membrane zone antibodies.
TAA was performed 7 days after discontinuation of all antibiotics. At that time, the skin lesions were in clinical remission. In brief, peripheral blood mononuclear cells were isolated using density gradient centrifugation. The cells were then incubated in culture media containing cefuroxime, moxifloxacin, piperacillin/tazobactam, teicoplanin, or solvent control. Culture supernatants were collected on days 7, 14, and 21, and the levels of cytokines granulysin and granzyme B were measured with Enzyme-Linked Immunosorbent Assay. The levels of granulysin and granzyme B were the highest in the piperacillin/tazobactam culture supernant (granulysin, 6 times higher than the solvent control and granzyme b, 31.2 times higher than the solvent control), followed by moxifloxacin (granulysin, 3.4 times higher than the solvent control and granzyme B, 7.7 times higher than the solvent control). There were no significant increases in the cytokine levels in the cefuroxime and teicoplanin culture supernants.
Based on the timing and duration of antibiotics usage, disease course, histopathological findings, and DIF, we suspected a diagnosis of drug induced-LABD most likely triggered by piperacillin-tazobactam. This was supported by the TAA results suggesting piperacillin/tazobactam as the main culprit drug. After cessation of the antibiotics, oral prednisolone 10 mg three times daily was begun, but was discontinued after one day because the patient developed tarry stools. Topical therapy included antimicrobial barrier dressings to the areas of erosion and clobetasol proprionate (0.05%) ointment to areas of intact erythematous skin. The patient's symptoms improved rapidly. At follow-up two weeks later, there was near complete resolution of all skin lesions.
LABD is a rare disease with an incidence of 0.23~2.3 cases per million person-years1. LABD has a bipolar age distribution, afflicting mostly children aged 6 months to 10 years or adults over 60 years old5.
Patients with LABD may have lesions on the skin and/or mucous membranes4, and typically present with a widespread skin eruption of vesicles and bullae on inflamed or non-inflamed skin1. The acute onset of annular or arcuate blisters resembling a string or cluster of pearls has been reported to strongly suggest a diagnosis of LABD34. The trunk, extensor extremities, buttocks, and perioral areas are the most common lesion sites in adults2. Unlike our patient, adult patients usually do not manifest lesions in the body folds and flexor areas3.
LABD is usually idiopathic but may be related to infection or medication5. Vancomycin is the most common medication reported to induce LABD15. Other implicated medications include penicillins, cephalosporins, angiotensin converting enzyme inhibitors, and nonsteroidal anti-inflammatory drugs5. However, penicillins have been rarely implicated in LABD. Piperacillin-tazobactam-induced LABD is less common, and only one case has been previously reported and that patient presented with Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) like findings1.
Cytotoxic mechanisms are involved in most forms of drug-induced skin disease911. Granulysin, a secretory protein produced by cytotoxic T lymphocytes and natural killer cells, plays a key role in disseminated keratinocyte death in SJS/TEN78 and various cutaneous adverse reactions (CAR)911. Other mediator, such as granzyme B, is expressed in epidermal T cells at the dermoepidermal junction and mediates drug-specific cytotoxicity in T-cell lines and clones derived from patients with different CARs911. Since increased concentrations of granulysin and granzyme B are significantly correlated with CARs789, a positive TAA result has been defined as a 2-fold increase in cytokine expression compared with that of the solvent control8. In our case, the TAA showed a strong reaction to piperacillin-tazobactam and provided evidence useful for identifying the causative drug. Larger studies of similar cases are necessary to justify the use of TAA for the detection of causative drugs.
The primary treatment of patients with drug-induced LABD is withdrawal of the offending agent5. Other accepted treatments include dapsone, sulfonamides (sulfapyridine and sulfamethoxypyridazine), colchicine, and topical and oral corticosteroids5. In our case, spontaneous resolution of the skin eruption several days after discontinuation of piperacillin-tazobactam strengthened our suspicion that piperacillin-tazobactam was the culprit drug. In conclusion, piperacillin-tazobactam can occasionally induce LABD. Unlike the first case report describing a patient with SJS/TEN-like skin lesions, our case presented with annular or arciform blisters mainly in the intertriginous and flexural areas. If possible, LTT or TAA should be performed to potentially identify the causative drug.
Figures and Tables
Fig. 1
(A~C) Photographs showing numerous clear fluid-filled tense bullae with erosions and crust formation on the axillae, thighs, groins and popliteal fossae; (D) some lesions are arciform in appearance.
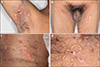
Fig. 2
Photomicrographs of the patient's cutaneous biopsy specimen demonstrate subepidermal blister with dense neutrophils, nuclear dust, and eosinophils in the dermis (A: H&E, ×40; B: H&E, ×100). Photomicrographs of direct immunofluorescent staining of the skin biopsy specimen demonstrated continuous linear deposition of IgA and weak linear deposition of C3 along the basement membrane zone (C, D: ×100).
ACKNOWLEDGMENT
We would like to thank Professor Wen-Hung Chung and his team for conducting the T-cell activation assay.
Funding by Industry-University Cooperative Research Project, Taiwan (R11004).
We received the patient consent form about publishing all photographic materials.
References
1. Adler NR, McLean CA, Aung AK, Goh MS. Piperacillin-tazobactam-induced linear IgA bullous dermatosis presenting clinically as Stevens-Johnson syndrome/toxic epidermal necrolysis overlap. Clin Exp Dermatol. 2017; 42:299–302.

2. Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012; 30:38–50.

3. Sakka N, Yahia KH, Volcon A, Brazilai A, Baum S. Intertriginous linear iga bullous dermatosis treated with colchicine. J Dermatol Clin Res. 2015; 3:1040.
4. Lings K, Bygum A. Linear IgA bullous dermatosis: a retrospective study of 23 patients in Denmark. Acta Derm Venereol. 2015; 95:466–471.

5. Jha P, Swanson K, Stromich J, Michalski BM, Olasz E. A rare case of vancomycin-induced linear immunoglobulin A bullous dermatosis. Case Rep Dermatol Med. 2017; 2017:7318305.

6. Tomida E, Kato Y, Ozawa H, Hasegawa H, Ishii N, Hashimoto T, et al. Causative drug detection by drug-induced lymphocyte stimulation test in drug-induced linear IgA bullous dermatosis. Br J Dermatol. 2016; 175:1106–1108.

7. Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008; 14:1343–1350.

8. Chung WH, Pan RY, Chu MT, Chin SW, Huang YL, Wang WC, et al. Oxypurinol-specific T cells possess preferential TCR clonotypes and express granulysin in allopurinol-induced severe cutaneous adverse reactions. J Invest Dermatol. 2015; 135:2237–2248.

9. Porębski G, Czarnobilska E, Bosak M. Cytotoxic-based assays in delayed drug hypersensitivity reactions induced by antiepileptic drugs. Pol Arch Med Wewn. 2015; 125:823–834.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download