Abstract
Dark circles refer to visible darkness of the infra-orbital areas. Dark circles are a cosmetic concern for many individuals, although not a medical concern. Moreover, clear definitions and possible causes of dark circles have not been elucidated. This study reviews the possible causes and treatment options for dark circles based on a review of the medical literature and the authors' clinical experience. Potential factors that contribute to dark circles include excessive pigmentation, shadowing due to tear troughs and infra-orbital fat herniation, shadowing due to infraorbital laxity and wrinkles, and thin, translucent skin overlying the orbicularis oculi muscle. Given the different possible causes for dark circles, therapeutic modalities must be individualized for each patient. Because various factors cause dark circles, it is useful to identify the underlying causes in order to select the appropriate treatment.
Cosmetic problems that are neither health-threatening nor associated with significant morbidity but can affect an individual's emotional well-being are gaining increased attention. “Dark circles” is not a formal medical term, but a widely used term that collectively indicates symptoms of dark areas under the eyes. Visible dark circles make people look tired and can be a significant cosmetic concern123. Many affected individuals seek treatment for dark circles, and this condition affects a wide range of ages, both sexes, and all races4. Despite the prevalence of this condition, there are few published articles on the mechanisms that underlie the conspicuousness of dark circles5. In addition, there is no clear consensus about the pathomechanism and standard treatment of dark circles. Herein the authors introduce and review the possible causes of infraorbital dark circles and individualized treatment options depending on the underlying cause, with a particular emphasis on injection methods.
Possible causative factors of infraorbital dark circles include many exogenous and endogenous factors, such as anatomical differences, sex, genetic predisposition, aging, general physical condition, atopic dermatitis, and dryness. There are several clinical causes of infraorbital dark circles: excessive pigmentation, tear troughs; shadowing due to infra-orbital fat herniation; thin, translucent skin overlying the orbicularis oculi muscle; running of the veins; and shadowing due to infraorbital laxity and wrinkles.
Infra-orbital dark circles are more common in patients with allergic conditions such as atopic dermatitis or allergic contact dermatitis (Fig. 1A). In these patients, periorbital dermatitis and pruritus-induced rubbing habits can cause postinflammatory hyperpigmentation (PIH) around the eyes. Dark circles under the eyes are often accompanied by mouth breathing in children with allergic rhinitis, which causes venous stasis due to impedance of blood flow through the edematous nasal mucosa6. Post-purpuric pigmentation (PPP) after a cosmetic procedure is another cause of periorbital darkness (Fig. 1B). PPP as a type of PIH is an acquired hypermelanosis induced by injury or cosmetic procedures, such as plastic surgery, lasers, or chemical peels, and it tends to affect dark-skinned people with greater frequency and severity.
There has been some debate regarding the contributing anatomy of tear trough deformity. Generally, the tear trough, also known as the nasojugal groove, is the natural depression extending inferolaterally from the medial canthus, while the lid/cheek junction, or palpebromalar groove, extends around the lateral half of the inferior orbit7. This concave, oblique groove can produce dark circles and make people look tired and aged (Fig. 2).
Infraorbital fat herniation itself does not directly create dark circles. However, if the infraorbital fat protrudes, the tear trough become deeper, and dark circles look worse (Fig. 3).
Transparent eyelid skin allows visualization of the underlying subcutaneous vascular plexus or vasculature within the muscle.
Wrinkles and laxity in infraorbital areas are other causes of dark circles and generally worsen with age (Fig. 4).
Despite the great number of available medications and therapies to treat infraorbital dark circles, there are no evidence-based studies to support their use. Given the different possible causes of dark circles, therapeutic modalities must be individualized for each patient.
A variety of topical treatments can be used to treat dermatitis-related PIH or PPP with varying degrees of success. Topical treatment options include bleaching agents such as hydroquinone and tretinoin and depigmenting agents such as albutin, an extract of the bearberry plant. Topical corticosteroids, topical antioxidants including vitamin C and E, kojic acid, and azelaic acid also have been prescribed8. Lightening of hyperpigmented areas can be achieved with one or more topical agents. Most topical agents can be effective but require a long period to achieve good results. In addition to long-term use, those agents can cause irritation or pruritus in some patients who apply them to the sensitive skin near the eyes. To overcome those disadvantages and increase the penetration rate of active ingredients, a novel hyaluronic acid microneedle patch system with depigmenting agents has been introduced9.
Currently, soft tissue augmentation with fillers is a widely accepted treatment for improving quality of life. This treatment can be used for correcting tear troughs, thin skin, and infraorbital wrinkles/laxity10. Dark circles due to tear troughs alone or tear troughs with infraorbital fat herniation are the most common type of clinically important dark circles. We can treat these types of dark circles with hyaluronic acid (HA) fillers; HA fillers were well tolerated in the periorbital region in a long-term study11.
Clinical experience and data have demonstrated that product choice and injection technique are critical features of safe and successful infraorbital filling12. Many fillers have recently been introduced, and selection criteria for fillers are diverse. Considerations when choosing fillers include:
Fillers can be generally divided into HA or non-HA fillers. When considering the ease of removal and possible side effects, it is appropriate to use HA fillers for tear troughs.
When manufacturers convey the concentration of a filler, they are articulating the total amount of HA found in the filler, typically expressed in mg/ml. Higher HA content in fillers tends to show better volumizing and retaining effects13. However, when treating the infra-orbital area, an excessive volumizing effect can result in puffy eyes and make it difficult for physicians to carefully control the injection depth. Therefore, low-concentration HA fillers are recommended for correcting dark circles.
HA is hydrated extensively by water at physiological pH. A HA gel's capacity for swelling will vary from product to product and is dependent upon concentration, cross-link density, and the process used to hydrate the gel1415. Physicians should consider the swelling capacity of the fillers to be used, so that overcorrection can be prevented.
The rheology of HA fillers is very important for treating dark circles. It is convenient to use fillers with relatively low elasticity and viscosity for the correction of dark circles16.
When characterizing the particle size of a HA gel, consideration must be given to the average particle size as well as the particle size distribution. Because larger gel particles are more difficult to push through a small-bore needle, a filler with a high average particle size will be more difficult to extrude14. It is important to control the size of the gel particles so as to reduce the extrusion force and related side effects, such as bleeding and pain, when gels are administered17. We recommend choosing a filler with the smallest particles possible when treating infra-orbital areas.
‘Tear trough alone’-type dark circles are seen frequently in young people, in most of whom dark circles can be improved by simply filling the tear trough. Tear troughs with prolapse of orbital fat due to aging can aggravate dark circles by indirectly causing shadowing over the lower lids. These cases can also be improved by filler injection, except in severe cases of infraorbital fat herniation. In tear troughs with anteromedial cheek depressions, simultaneous correction of both conditions can produce satisfactory results with regard to dark circles. Perfection of the midfacial arc allows for a brighter and younger looking face (Fig. 5).
To fill tear troughs, HA filler is injected using the linear thread method (three injections, 0.07 ml per injection). It is better to inject filler into the deep, submuscular layer to avoid a puffy appearance and the Tyndall phenomenon. The infraorbital area has been empirically divided into zones 1 to 5 (Fig. 6). A small bolus HA injection using the depot technique can be given at the point of the infraorbital area (5 small boluses, 0.02 ml per injection, 0.1 ml in total per side).
When treating the infraorbital area with filler, physicians usually do not inject filler directly into the eyebag just beneath the eyelashes because there is concern about unnaturally swollen-looking eyes. However, HA fillers can be used not only for correction of tear troughs, but also for rejuvenation of eyebag areas (Fig. 7). It is possible for HA fillers to improve hydration, collagen synthesis, and the antioxidant effect, in addition to having a simple volumizing effect. We suggest that dermal rejuvenation with HA fillers is appropriate for treating wrinkles, texture changes, and dyschromia of the eyebag areas18. Successful correction of the lower eyelid depends on patient selection, precise and consistent injection technique, and selection of appropriate filler material. In these cases, most light HA products such as Restylane® vital, Belotero® soft, and Juvederm® volbella are recommended for use.
Tear troughs and visualization of the underlying vasculature within the muscle through thin and transparent lower eyelid skin can be improved by autologous fat transplantation. Fat transplantation is less likely to lead to a puffy appearance or the Tyndall effect compared to filler treatment. However, the procedure takes a longer time, edema can last for long periods, and removal is difficult.
Platelet-rich plasma (PRP) is a concentration of plasma protein derived from a patient's own blood that has been centrifuged to remove red blood cells. It has a significant concentration of growth factors, chemokines, and cytokines and has been used to encourage various healing process. There has been a recent increase in the application of PRP in dermatology and aesthetic fields, including sound healing, fat grafting, alopecia, scar revision, and dermal volume augmentation1920.
PRP treatment is mainly effective for wrinkles, laxity, and secondary PIH-related dark circles. PRP can stimulate dermal fibroblast proliferation and collagen synthesis21. Transforming growth factor-β1 and epidermal growth factor in PRP are suggested to inhibit melanin production via delayed extracellular signal-regulated kinase activation and inhibition of prostagandin-E2 expression/tyrosinase enzyme activity, respectively2223. In addition, PRP improves fat graft survival and can be used in combination with autologous fat grafts for dark circles24. For the treatment of dark circles, 0.01~0.015 ml PRP is intradermally multi-injected with 30-gauge needles at a single point over each lower eyelid.
Intradermal long-chain polynucleotide (PN) filler injection appears to be an effective and safe treatment for dark circles due to thin and sagging skin. High-molecular weight chained PNs are thought to mediate their effects primarily through an increase in the metabolic activity of fibroblasts, which stimulate the secretion of collagen proteins and control the renewal of various dermal components. PNs contribute to the regeneration of several key autologous components of the skin, such as glycosaminoglycans, proteins, glycoproteins, and fibrils, which help to maintain physiological function2526. In a comparison study with HA fillers, PN fillers showed greater increases in skin elasticity and collagen synthesis followed by fibroblast stimulation compared to HA filler groups with similar durability. PN fillers showed similarly good efficacy and safety in the correction of crow's feet in a clinical study27.
In treating our patients, injection of a PN filler (Rejuran®; Pharmareasearch Products, Inc., Seongnam, Korea) is performed intradermally with a 33-gauge nanoneedle using a serial puncture technique, with injections placed approximately 1 cm apart over both infraorbital areas. The total dose is about 0.1~0.5 ml for each side. Approximately 0.01~0.02 ml of material is contained in each injection, and a total of 12~24 points were injected into each infraorbital area. Lumps appear immediately after treatment but usually disappear spontaneously after 1~2 days. Fig. 8 shows improvement of dark circles with thin skin lacking elasticity after PN filler injection.
Lasers will resurface the skin and reduce or eliminate wrinkles and pigmentation, which improves the overall appearance of infraorbital dark circles. Various lasers can be used for treatment of dark circles. A low-fluence Q-switched 1,064-nm laser is safe and effective for dark circles with excessive pigmentation28. Fractional lasers and radiofrequency devices might be suitable for dark circles with loose and wrinkled skin2930. Other reports have shown that 2790-nm Er:YSGG laser therapy might be effectively and safely used for the treatment of infraorbital dark circles in atopic dermatitis patients31. In addition, a long-pulsed 1,064-nm neodymium-doped yttrium aluminum garnet laser can be used for the treatment of venous infraorbital dark circles and selectively minimizes visible prominent veins3233.
Transconjunctival infraorbital fat resection and transposition can soften tear trough deformities and correct infraorbital fat herniation34. Laser-assisted transconjunctival lower lid blepharoplasty is also a safe and low-risk procedure that produces excellent cosmetic results in infraorbital fat pads35. Injectable phosphatidylcholine can be another option for infraorbital fat herniation that might benefit some patients who are considering blepharoplasty36; however, phosphatidylcholine is not approved in the United States for fat elimination. There are currently no Food and Drug Administration-approved injectable drugs for fat elimination37.
Because various factors cause dark circles, it is necessary to identify the cause before appropriate treatment can be initiated. Individual patients might have more than a single underlying cause, so physicians should be familiar with diverse treatment options and manage dark circles on a case-by-case basis. Treatment modalities range from topical agents to non-invasive treatments such as lasers to more invasive treatments such as volume augmentation with HA fillers or fat transplantation. Because filler injections in the infraorbital area can yield satisfactory results, we shared our experience. Dermatologists can choose the above-mentioned treatment options alone or in combination to individualize treatment.
Figures and Tables
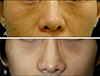 | Fig. 1Infraorbital darkness due to excessive pigmentation. (A) Chronic rubbing-induced postinflammatory hyperpigmentation in an atopic dermatitis patient. (B) Post-purpuric pigmentation around the eyes after two-jaw surgery. We received the patient's consent form about publishing all photographic materials. |
 | Fig. 5(A, C) Dark circles due to both tear troughs and malar retraction. (B, D) Improvement in dark circles with correction of tear troughs and mid-facial depressions with hyaluronic acid filler. Dotted lines indicate the contour of the anteromedial cheek. |
ACKNOWLEDGMENT
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministary of Health & Welfare, Republic of Korea (HN14C0095).
References
1. Nkengne A, Bertin C, Stamatas GN, Giron A, Rossi A, Issachar N, et al. Influence of facial skin attributes on the perceived age of Caucasian women. J Eur Acad Dermatol Venereol. 2008; 22:982–991.

2. Camp MC, Wong WW, Filip Z, Carter CS, Gupta SC. A quantitative analysis of periorbital aging with three-dimensional surface imaging. J Plast Reconstr Aesthet Surg. 2011; 64:148–154.

3. Mayes AE, Murray PG, Gunn DA, Tomlin CC, Catt SD, Wen YB, et al. Ageing appearance in China: biophysical profile of facial skin and its relationship to perceived age. J Eur Acad Dermatol Venereol. 2010; 24:341–348.

4. Freitag FM, Cestari TF. What causes dark circles under the eyes? J Cosmet Dermatol. 2007; 6:211–215.

5. Roh MR, Chung KY. Infraorbital dark circles: definition, causes, and treatment options. Dermatol Surg. 2009; 35:1163–1171.

6. Marks MB. Allergic shiners. Dark circles under the eyes in children. Clin Pediatr (Phila). 1966; 5:655–658.
7. Haddock NT, Saadeh PB, Boutros S, Thorne CH. The tear trough and lid/cheek junction: anatomy and implications for surgical correction. Plast Reconstr Surg. 2009; 123:1332–1340. discussion 1341-1342.

9. Park KY, Kwon HJ, Lee C, Kim D, Yoon JJ, Kim MN, et al. Efficacy and safety of a new microneedle patch for skin brightening: a randomized, split-face, single-blind study. J Cosmet Dermatol. 2017; 16:382–387.

10. Sharad J. Dermal fillers for the treatment of tear trough deformity: a review of anatomy, treatment techniques, and their outcomes. J Cutan Aesthet Surg. 2012; 5:229–238.

11. Mustak H, Fiaschetti D, Goldberg RA. Filling the periorbital hollows with hyaluronic acid gel: long-term review of outcomes and complications. J Cosmet Dermatol. 2017; [Epub ahead of print]. DOI: 10.1111/jocd.12452.

12. Sundaram H, Cassuto D. Biophysical characteristics of hyaluronic acid soft-tissue fillers and their relevance to aesthetic applications. Plast Reconstr Surg. 2013; 132:4 Suppl 2. 5S–21S.

13. Pierre S, Liew S, Bernardin A. Basics of dermal filler rheology. Dermatol Surg. 2015; 41:Suppl 1. S120–S126.

14. Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009; 35:Suppl 1. 302–312.

15. Park S, Park KY, Yeo IK, Cho SY, Ah YC, Koh HJ, et al. Investigation of the degradation-retarding effect caused by the low swelling capacity of a novel hyaluronic acid filler developed by solid-phase crosslinking technology. Ann Dermatol. 2014; 26:357–362.

16. Park KY, Kim HK, Kim BJ. Comparative study of hyaluronic acid fillers by in vitro and in vivo testing. J Eur Acad Dermatol Venereol. 2014; 28:565–568.

17. Stocks D, Sundaram H, Michaels J, Durrani MJ, Wortzman MS, Nelson DB. Rheological evaluation of the physical properties of hyaluronic acid dermal fillers. J Drugs Dermatol. 2011; 10:974–980.
18. Park KY, Seok J, Ko EJ, Kim BJ, Kim MN, Youn CS. Hyaluronic acid filler combined with antioxidants for infraorbital rejuvenation: report of two cases. Dermatol Ther. 2017; 30:DOI: 10.1111/dth.12448.

19. Al-Shami SH. Treatment of periorbital hyperpigmentation using platelet-rich plasma injections. Am J Dermatol Venereol. 2014; 3:87–94.
20. Mehryan P, Zartab H, Rajabi A, Pazhoohi N, Firooz A. Assessment of efficacy of platelet-rich plasma (PRP) on infraorbital dark circles and crow's feet wrinkles. J Cosmet Dermatol. 2014; 13:72–78.

21. Kim DH, Je YJ, Kim CD, Lee YH, Seo YJ, Lee JH, et al. Can platelet-rich plasma be used for skin rejuvenation? Evaluation of effects of platelet-rich plasma on human dermal fibroblast. Ann Dermatol. 2011; 23:424–431.

22. Kim DS, Park SH, Park KC. Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal regulated kinase activation. Int J Biochem Cell Biol. 2004; 36:1482–1491.
23. Yun WJ, Bang SH, Min KH, Kim SW, Lee MW, Chang SE. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013; 39:1903–1911.

24. Oh DS, Cheon YW, Jeon YR, Lew DH. Activated platelet-rich plasma improves fat graft survival in nude mice: a pilot study. Dermatol Surg. 2011; 37:619–625.

25. Sini P, Denti A, Cattarini G, Daglio M, Tira ME, Balduini C. Effect of polydeoxyribonucleotides on human fibroblasts in primary culture. Cell Biochem Funct. 1999; 17:107–114.

26. Park KY, Seok J, Rho NK, Kim BJ, Kim MN. Long-chain polynucleotide filler for skin rejuvenation: efficacy and complications in five patients. Dermatol Ther. 2016; 29:37–40.

27. Pak CS, Lee J, Lee H, Jeong J, Kim EH, Jeong J, et al. A phase III, randomized, double-blind, matched-pairs, activecontrolled clinical trial and preclinical animal study to compare the durability, efficacy and safety between polynucleotide filler and hyaluronic acid filler in the correction of crow's feet: a new concept of regenerative filler. J Korean Med Sci. 2014; 29:Suppl 3. S201–S209.

28. Xu TH, Yang ZH, Li YH, Chen JZ, Guo S, Wu Y, et al. Treatment of infraorbital dark circles using a low-fluence Q-switched 1,064-nm laser. Dermatol Surg. 2011; 37:797–803.

29. Sukal SA, Chapas AM, Bernstein LJ, Hale EK, Kim KH, Geronemus RG. Eyelid tightening and improved eyelid aperture through nonablative fractional resurfacing. Dermatol Surg. 2008; 34:1454–1458.

30. Lolis MS, Goldberg DJ. Assessment of safety and efficacy of a bipolar fractionated radiofrequency device in the treatment of periorbital rhytides. J Cosmet Laser Ther. 2014; 16:161–164.

31. Park KY, Oh IY, Moon NJ, Seo SJ. Treatment of infraorbital dark circles in atopic dermatitis with a 2790-nm erbium: yttrium scandium gallium garnet laser: a pilot study. J Cosmet Laser Ther. 2013; 15:102–106.

32. Chen DL, Cohen JL. Treatment of periorbital veins with long-pulse Nd:YAG laser. J Drugs Dermatol. 2015; 14:1360–1362.
33. Ma G, Lin XX, Hu XJ, Jin YB, Chen H. Treatment of venous infraorbital dark circles using a long-pulsed 1,064-nm neodymium-doped yttrium aluminum garnet laser. Dermatol Surg. 2012; 38:1277–1282.

34. Grant JR, Laferriere KA. Periocular rejuvenation: lower eyelid blepharoplasty with fat repositioning and the suborbicularis oculi fat. Facial Plast Surg Clin North Am. 2010; 18:399–409.

35. Kim SW, Kim WS, Cho MK, Whang KU. Transconjunctival laser blepharoplasty of lower eyelids: Asian experience with 1,340 cases. Dermatol Surg. 2003; 29:74–79.

36. Ablon G, Rotunda AM. Treatment of lower eyelid fat pads using phosphatidylcholine: clinical trial and review. Dermatol Surg. 2004; 30:422–427.

37. U.S. Food and Drug Administration. FDA Information on Lipodissolve [Internet]. Silver Spring, MD: U.S. Food and Drug Administration;update 2010 Apr 7. cited 2018 Feb 6. Available from: http://wayback.archive-it.org/7993/20171115004713/https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompounding/ucm207624.htm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download