1. Merskey H, Bogduk N. Classification of Chronic Pain. 2nd ed. Seattle: International Association for the Study of Pain Press;1994.
2. Arora M, Sangwan P, Tewari S, Duhan J. Effect of maintaining apical patency on endodontic pain in posterior teeth with pulp necrosis and apical periodontitis: A randomized controlled trial. Int Endod J. 2016; 49:317–324. PMID:
25866134.

3. Sathorn P, Parashos P, Messer H. The prevalence of postoperative pain and flare-up in single- and multiple-visit endodontic treatment: a systematic review. Int Endod J. 2008; 41:91–99. PMID:
17956561.

4. Zuckerman O, Metzger Z, Sela G, Lin S. [“Flare-up” during endodontic treatment--etiology and management ]. Refuat Hapeh Vehashinayim. 1993; 24:19–26.
5. Rosenberg PA. Clinical strategies for managing endodontic pain. Endod Top. 2002; 3:78–92.

6. Haas DA. Local and systemic therapeutics for the control of endodontic pain. Alpha Omegan. 1997; 90:73–76. PMID:
10634107.
7. Stewart GG. Antihistamines and corticosteroids in the reduction of postoperative sequelae following endodontic surgery. Oral Surg Oral Med Oral Pathol. 1956; 9:216–220. PMID:
13297381.
8. Alexander RE, Throndson RR. A review of perioperative corticosteroid use in dentoalveolar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000; 90:406–415. PMID:
11027375.

9. Kidd BL, Urban LA. Mechanisms of inflammatory pai. Br J Anaesth. 2001; 87:3–11. PMID:
11460811.
10. Ingawale DK, Mandlik SK, Patel SS. An emphasis on molecular mechanisms of anti-inflammatory effects and glucocorticoid resistance. J Complement Integr Med. 2015; 12:1–13. PMID:
25503867.

11. Newton R. Anti-inflammatory glucocorticoids: Changing concepts. Eur J Pharmacol. 2014; 724:231–236. PMID:
23747654.

12. Ricciotti E, Fitzgerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011; 31:986–1000. PMID:
21508345.
13. Becker DE. Basic and clinical pharmacology of autonomic drugs. Anesth Prog. 2012; 59:159–168. PMID:
23241039.

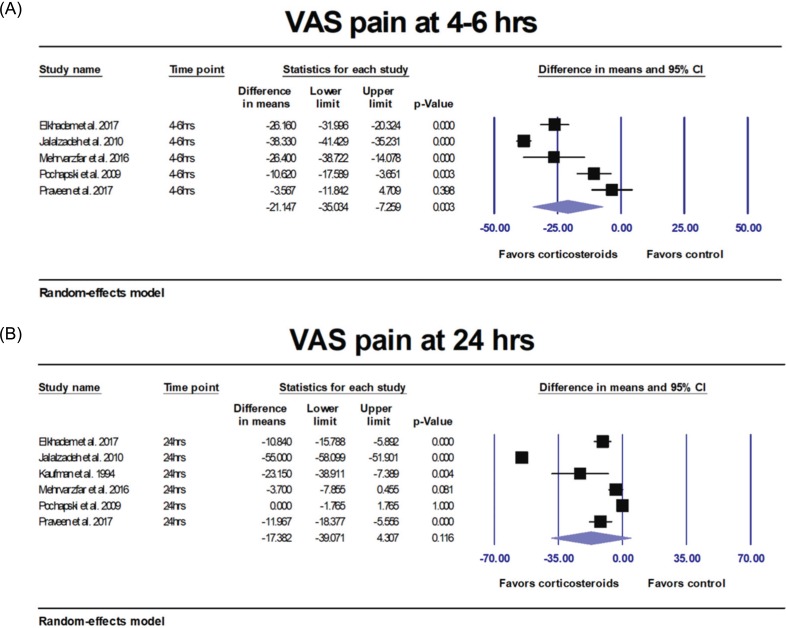
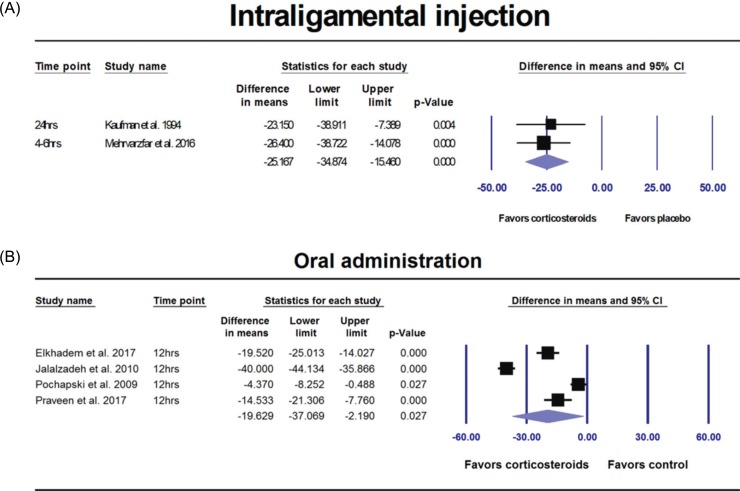
14. Elkhadem A, Ezzat K, Ramadan M, Abdelghaffar S, Khamis D, Hassan A, et al. The effect of preoperative oral administration of prednisolone on postoperative pain in patients with symptomatic irreversible pulpitis: A single-centre randomized controlled trial. Int Endod J. 2018; 51:e189–e196. PMID:
28560802.

15. Praveen R, Thakur S, Kirthiga M. Comparative Evaluation of Premedication with Ketorolac and Prednisolone on Postendodontic Pain: A Double-blind Randomized Controlled Trial. J Endod. 2017; 43:667–673. PMID:
28320541.

16. Nogueira BML, Silva LG, Mesquita CRM, Menezes SAF, Menezes TOA, Faria AGM, et al. Is the Use of Dexamethasone Effective in Controlling Pain Associated with Symptomatic Irreversible Pulpitis? A Systematic Review. J Endod. 2018; 44:703–710. PMID:
29571913.

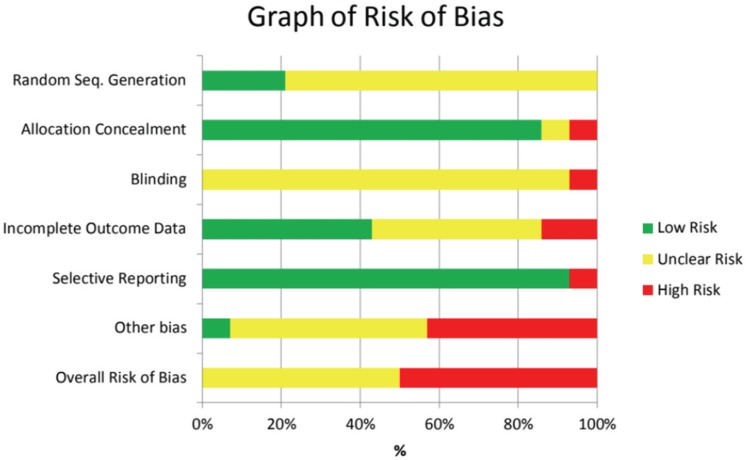
17. Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. updated March 2011.
18. Cochran W. The combination of estimates from different experiments. Biometrics. 1954; 10:101–129.

19. Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558. PMID:
12111919.

20. Mehrvarzfar P, Esnashari E, Salmanzadeh R, Fazlyab M, Fazlyab M. Effect of dexamethasone intraligamentary injection on post-endodontic pain in patients with symptomatic irreversible pulpitis: A randomized controlled clinical trial. Iran Endod J. 2016; 11:261–266. PMID:
27790253.
21. Pochapski MT, Santos FA, de Andrade ED, Sydney GB. Effect of pretreatment dexamethasone on postendodontic pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108:790–795. PMID:
19748294.

22. Rogers MJ, Johnson BR, Remeikis NA, BeGole EA. Comparison of effect of intracanal use of ketorolac tromethamine and dexamethasone with oral ibuprofen on post treatment endodontic pain. J Endod. 1999; 25:381–384. PMID:
10530266.

23. Shantiaee Y, Mahjour F, Dianat O. Efficacy comparison of periapical infiltration injection of dexamethasone, morphine and placebo for postoperative endodontic pain. Int Dent J. 2012; 62:74–78. PMID:
22420475.

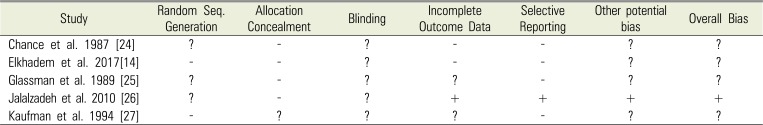
24. Chance K, Lin L, Shovlin FE, Skribner J. Clinical trial of intracanal corticosteroid in root canal therapy. J Endod. 1987; 13:466–468. PMID:
3328770.

25. Glassman G, Krasner P, Morse DR, Rankow H, Lang J, Furst ML. A prospective randomized double-blind trial on efficacy of dexamethasone for endodontic interappointment pain in teeth with asymptomatic inflamed pulps. Oral Surg Oral Med Oral Pathol. 1989; 67:96–100. PMID:
2643074.

26. Jalalzadeh SM, Mamavi A, Shahriari S, Santos FA, Pochapski MT. Effect of pretreatment prednisolone on postendodontic pain: A double-blind parallel-randomized clinical trial. J Endod. 2010; 36:978–981. PMID:
20478449.

27. Kaufman E, Heling I, Rotstein I, Friedman S, Sion A, Moz C, et al. Intraligamentary injection of slow-release methylprednisolone for the prevention of pain after endodontic treatment. Oral Surg Oral Med Oral Pathol. 1994; 77:651–654. PMID:
8065733.

28. Krasner P, Jackson E. Management of posttreatment endodontic pain with oral dexamethasone: a double-blind study. Oral Surg Oral Med Oral Pathol. 1986; 62:187–190. PMID:
3528979.

29. Liesinger A, Marshall FJ, Marshall JG. Effect of variable doses of dexamethasone on posttreatment endodontic pain. J Endod. 1993; 19:35–39. PMID:
8289025.

30. Marshall JG, Walton RE. The effect of intramuscular injection of steroid on posttreatment endodontic pain. J Endod. 1984; 10:584–588. PMID:
6596389.

31. Mehrvarzfar P, Shababi B, Sayyad R, Fallahdoost A, Kheradpir K. Effect of supraperiosteal injection of dexamethasone on postoperative pain. Aust Endod J. 2008; 34:25–29. PMID:
18352900.

32. Aggarwal V, Singla M, Rizvi A, Miglani S. Comparative evaluation of local infiltration of articaine, articaine plus ketorolac, and dexamethasone on anesthetic efficacy of inferior alveolar nerve block with lidocaine in patients with irreversible pulpitis. J Endod. 2011; 37:445–449. PMID:
21419287.

33. Marshall JG, Liesinger AW. Factors associated with endodontic posttreatment pain. J Endod. 1993; 19:573–575. PMID:
8151248.

34. Kelly A-M. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001; 18:205–207. PMID:
11354213.

35. Lexi-Comp Online for Dentistry I. Lexi-Comp Online for Dentistry, Inc.;2018.
36. Patten JR, Patten J, Hutchins MO. Adjunct use of dexamethasone in postoperative dental pain control. Compendium. 1992; 13:580. 582. 584. PMID:
1521294.
37. Mohammadi Z. Systemic and local applications of steroids in endodontics: an update review. Int Dent J. 2009; 59:297–304. PMID:
19998665.
38. Iranmanesh F, Parirokh M, Haghdoost AA, Abbott PV. Effect of Corticosteroids on Pain Relief Following Root Canal Treatment: A Systematic Review. Iran Endod J. 2017; 12:123–130. PMID:
28496516.
39. Steyaert A, De Kock M. Chronic postsurgical pain. Curr Opin Anaesthesiol. 2012; 25:584–588. PMID:
22895123.

40. Fletcher MC, Spera JF. Management of Acute Postoperative Pain after Oral Surgery. Dent Clin North Am. 2012; 56:95–111. PMID:
22117944.

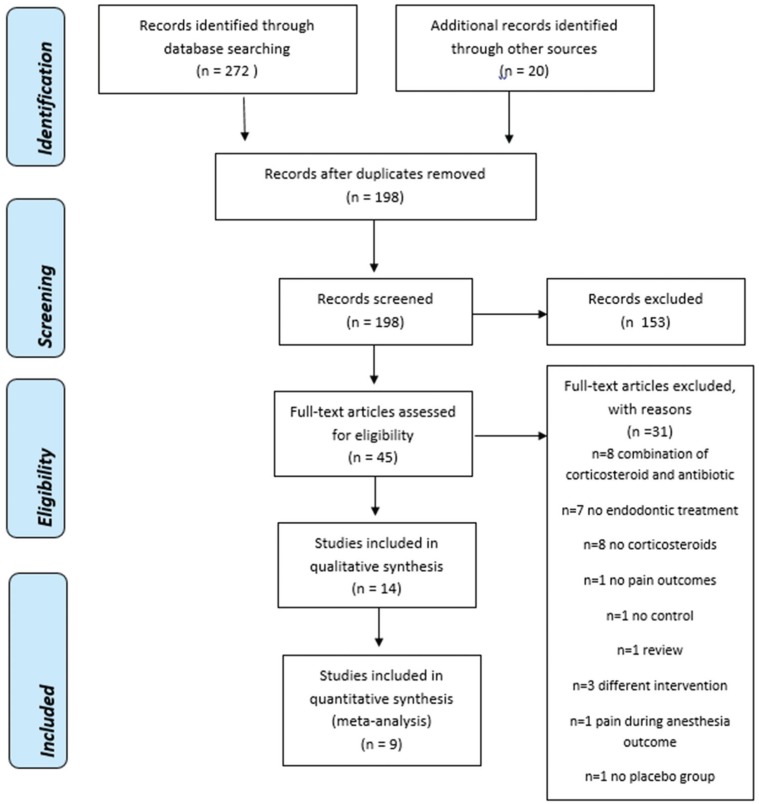
41. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097. PMID:
19621072.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download