Abstract
Acute kidney injury (AKI), which is defined as a rapid decline of renal function, becomes common and recently recognized to be closely intertwined with chronic kidney diseases. Current treatment for AKI is largely supportive, and endoplasmic reticulum (ER) stress has emerged as a novel mediator of AKI. Since carbon monoxide attenuates ER stress, the objective of the present study aimed to determine the protective effect of carbon monoxide releasing molecule-2 (CORM2) on AKI associated with ER stress. Kidney injury was induced after LPS (15 mg/kg) treatment at 12 to 24 h in C57BL/6J mice. Pretreatment of CORM2 (30 mg/kg) effectively prevented LPS-induced oxidative stress and inflammation during AKI in mice. CORM2 treatment also effectively inhibited LPS-induced ER stress in AKI mice. In order to confirm effect of CO on the pathophysiological role of tubular epithelial cells in AKI, we used mProx24 cells. Pretreatment of CORM2 attenuated LPS-induced ER stress, oxidative stress, and inflammation in mProx24 cells. These data suggest that CO therapy may prevent ER stress-mediated AKI.
Acute kidney injury (AKI) is responsible for about 2 million deaths each year worldwide, and its incidence is rising [1]. In contrast to the traditional belief that survivors of AKI tend to fully recover renal function, there is growing evidence that patients who survive an episode of AKI might have a significant risk of developing progressive chronic kidney diseases [23]. Interventions to prevent poor patient outcomes during AKI are urgently needed, beside detecting AKI at early stages [4].
Endoplasmic reticulum (ER) stress [567] plays an important role in pathogenesis of AKI, along with reactive oxygen species (ROS) [89], apoptosis [10] and inflammation [11]. During ER stress, the activated unfolded protein response (UPR) is associated with RNA-dependent protein kinase-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1). The endoribonuclease activity of IRE1 cleaves XBP1 to generate spliced XBP1 (sXBP1), a potent transcription factor [12]. Active XBP1 in turn induces the expression of UPR target genes, and stimulates the production of inflammatory cytokine genes [13]. IRE1 is also responsible for activation of JNK during ER stress [14]. Additionally, ROS generation is associated with ER stress [1516]. Therefore, targeted inhibition of ER stress may hold promise as a new strategy in the fight against AKI.
Carbon monoxide (CO) is an endogenously produced gas resulting from the degradation of heme by heme oxygenase. While high concentration of CO represents a toxic inhalation hazard, low-dose of CO (~250–500 parts per million) shows protective functions in preclinical models of human diseases [17]. For instance, inhalation of CO attenuates inflammation and apoptosis in acute lung injury, ischemia reperfusion (I/R) injury in liver/ lung/heart, vascular injury and graft rejection [18]. CO also attenuates oxidative stress-induced inflammation and fibrosis in the hypoxic lungs [19]. CO or CO releasing molecule-2/3 (CORM2/3) inhibits ER stress in endothelial cells, splenic macrophages, hepatocytes, fibroblasts and ilets [2021]. In the kidney, CO exerts protective effects against fibrosis induced by unilateral ureteral obstruction in mice [22]. In addition, CO/CORMs can be beneficial for treating AKI induced by endotoxin or sepsis [2324], cisplatin [25], transplantation [26] as well as ischemia-reperfusion [2728] by suppressing oxidative injury, reducing cell apoptosis, and increasing cell survival. Although the protective effect of CO has been studied in AKI, the detailed mechanism remains elusive.
However, there is no study on effect of CO on ER stress in AKI. Since that ER stress has emerged as a novel biomarker for AKI and that CO therapy is a potential candidate for treatment of AKI, the current study aimed to determine whether CO therapy attenuates AKI through suppression of ER stress. We used lipopolysaccharide (LPS)-induced AKI among AKI mouse models such as LPS, cisplatin, cecal ligation and puncture (CLP), and ischemia reperfusion injury (IRI), since LPS model mimics many features of sepsis in human [293031], decreases glomerular filtration rate (GFR), increases blood urea nitrogen (BUN), and increases neutrophil infiltration [32].
Chemicals and antibodies were obtained from Sigma-Aldrich Company (St. Louis, MO, USA) and Cell Signaling Technology (Danvers, MA, USA), respectively, unless otherwise stated.
Six-week-old male C57BL/6 mice (Japan SLC Inc., Hamamatsu, Japan) were used in this study. In 1st series, mice were divided into four groups: (i) control, (ii) LPS 6 h, (iii) LPS 12 h, and (iv) LPS 24 h. Induction of AKI was evaluated at 6, 12, and 24 h with single intraperitoneal (i.p.) injection of LPS (15 mg/kg) [33]. In 2nd series, mice were divided into four groups: (i) control, (ii) CORM2, (iii) LPS, and (iv) LPS treated with CORM2. AKI was induced by single i.p. injection of 15 mg/kg LPS for 18 h. Age-matched control mice were injected with an equivalent volume of saline as vehicle of LPS. CORM2 at 30 mg/kg was administered (i.p.) to mice 2 h before administration (i.p.) of LPS, and age-matched control mice were injected with an equivalent volume of dimethyl sulfoxide (DMSO) as vehicle of CORM2 as described [33]. Mice were monitored at every 2 h interval during the experimental period. All mice were sacrificed at the above mentioned time point after LPS injection via anesthesia with 16.5% urethane (10 ml/kg). All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC No. 14-051) of Ewha Womans University.
Blood samples were collected with a heparinized syringe before mice were sacrificed. Plasma creatinine level was determined using a Detect X Serum Creatinine Detection Kit (Arbor Assays, Ann Arbor, MI, USA. Cystatin C level was measured using a cystatin C Elisa kit (R&D systems, Minneapolis, MN, USA). Plasma LPO level was measured by reacting with thiobarbituric acid as described previously [34].
LPO in kidney tissue was measured with the LPO assay kit (Cayman Chemical Co, Ann Arbor, MI, USA) according to the manufacturer's recommended protocols.
Frozen section of kidney tissues from AKI mice treated with or without CORM2 were used. Dihydroethidium (DHE) (5 µM, Molecular Probes, Eugene, Oregon, USA) was applied to the frozen section of kidneys (5 µm) for 10 min at 37℃ to reveal the presence of ROS with red fluorescence at 561 nm followed by DAPI staining and images were taken using a Zeiss ApoTome Axiovert 200M microscope (Carl Zeiss Microscopy GmbH, 07745 Jena, Germany).
For immunohistochemistry, we used anti-F4/80 (1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and anti-nitrotyrosine (1:200; Santa-Cruz Biotechnology) antibodies. Images were captured using a Zeiss microscope equipped with an Axio Cam HRC digital camera and Axio Cam software (Carl Zeiss, Thornwood, NY, USA). Staining intensities were then quantified using Image-Pro Plus 4.5 software (Media 149 Cybernetics, Silver Springs, MD, USA) as described previously [35].
Immortalized mProx24, mouse proximal tubular epithelial cells were cultured as described previously [36]. Subconfluent cells were pretreated with CORM2 (0, 5, 10, and 20 µM, dissolved in DMSO) for 2 h and then, stimulated with LPS (100 ng/ml, dissolved in distilled water) for 6 h.
Total RNA was extracted using Trizol reagent (Life Technologies, Carlsbad, CA, USA), and mRNA expression was measured by means of real-time PCR using an ABI7300 system (Applied Biosystems, Carlsbad, CA, USA) and 20 µl reaction volumes containing cDNA transcripts, primer pairs, and SYBR Green PCR Master Mix (Applied Biosystems) as described previously [35]. Primer sequences are shown in Table 1.
pNF-κB p65, NF-κB, pJNK, JNK, CHOP, and β-actin proteins were measured by standard western blot analysis as described previously [35] using anti-pNF-κB p65 (1:5000, Cell Signaling Technology), anti-NF-κB (1:4000, Cell Signaling Technology), anti-pJNK (1:5000, Cell Signaling Technology), anti-JNK (1:3000, Cell Signaling Technology), anti-CHOP (1:1000, Santa-Cruz Biotechnology), and anti-β-actin (1:1000) antibodies.
First, our study showed that time dependent treatment of LPS significantly decreased kidney weight to body weight (Fig. 1A) as well as increased markers of kidney injury such as plasma creatinine (Fig. 1B) and cystatin C (Fig. 1C). Though plasma cystatin C started to increase at 6 h, plasma creatinine increased at 12 h and both of them reached apparently at pick level at 24 h. Considering plasma creatinine, cystatin C or body condition, we decided 18 h time point for optimum AKI induction for further study. Pretreatment of CORM2 significantly reduced LPS-induced plasma creatinine (Fig. 1D) and cystatin C (Fig. 1E) levels, indicating that CORM2 may improve kidney function in mice. Then, we examined the tubular damage by analyzing the expression of kidney injury molecule (KIM1) levels. LPS-induced AKI mice showed increased plasma KIM1 levels (Fig. 1F) while CORM2 treatment significantly inhibited the effects (Fig. 1F).
As shown in Fig. 2A, the master regulator of inflammation, pNF-κB markedly increased by LPS while CORM2 significantly decreased the effects in the kidneys (Figs. 2A and B). Macrophage infiltration as measured by F4/80 staining was remarkably increased by LPS and CORM2 treatment significantly decreased the effects in the kidneys (Figs. 2C and D). As expected, mRNAs of LPS-induced proinflammatory cytokines such as TNFα, MCP1, iNOS, and ICAM1 were significantly inhibited by CORM2 in the kidneys (Fig. 2E). Since pathophysiological role of tubular epithelial cells in AKI has been implicated [3738], we used mProx cells in our study to confirm the effect of CORM2. Consistently, mRNAs of LPS-induced proinflammatory cytokines were also significantly inhibited by CORM2 in the mProx cells (Fig. 2F).
Since ER stress has emerged as a novel biomarker of AKI, we examined the effect of CORM2 on the downstream signaling of ER stress. Interestingly, CORM2 significantly decreased LPS-induced p-JNK (Figs. 3A and B) and CHOP (Figs. 3C and D) protein expression in the kidneys. In addition, mRNAs of ER stress markers such as spliced X-box-binding protein 1 (sXBP1), ER-degradation-enhancing-α-mannidose-like protein1 (Edem1), and glucose-regulated protein 78 (GRP78) were significantly attenuated by CORM2 in the AKI kidneys (Fig. 3E). Further to confirm the effect of CORM2 on ER stress markers, we used mProx cells. Consistently, mRNAs of LPS-induced ER stress markers such as sXBP1, Edem1, and GRP78 were also significantly inhibited by CORM2 in the mProx cells (Fig. 3F).
To confirm the anti-oxidative effect of CORM2, we measured plasma and kidney lipid hydroperoxide (LPO). Interestingly, CORM2 significantly inhibited LPS-induced plasma and kidney LPO (Figs. 4A and B). LPS-induced nitrotyrosine accumulation was also significantly decreased by CORM2 treatment in the kidney tissue (Fig. 4C). In addition, DHE staining was performed to directly measure ROS in kidney tissues. LPS effectively induced ROS generation which was significantly inhibited by CORM2 treatment (Fig. 4D). The mRNA levels of antioxidant genes such as catalase and Prx1 were inhibited by LPS while CORM2 treatment significantly increased their expression (Figs. 4E and F). CO is known to increase antioxidants such as NRF2, HO1, and NQO1. Consistently, mRNA levels of NRF2, HO1, and NQO1 were significantly increased by CORM2 in LPS-treated kidneys in mice (Fig. 4G). Consistently, the mRNA levels of NRF2, HO1, and NQO1 were also significantly increased by different doses of CORM2 in LPS treated mProx cells (Fig. 4H).
The present data demonstrated that administration of CORM2 could exert a protective effect against LPS-induced AKI by inhibiting ER stress.
LPS induces systemic inflammation which mimics many features of sepsis in human [293031]. In addition, LPS administration is simple and sterile [31] which are the important parameters for an experimental model. With respect to AKI, LPS injection causes decreased GFR, increased BUN, and increased neutrophil infiltration [32]. Thus, we employed LPS-induced AKI model in this study.
Protective effect of CORM2 on LPS-induced AKI is consistent with the previous studies [232425262728]. CORM2 protects CLP [24]- and IRI [27]-induced decreased GFR and inflammation in rats and mice. In addition, CO/CORMs inhibits AKI induced by endotoxin [23], cisplatin [25], and transplantation [26] by suppressing oxidative injury, and reducing cell apoptosis. In addition, high pressure CO preserves rat kidney graft from apoptosis and inflammation [39].
CO is known to inhibit ER stress in different cell types including endothelial cells, splenic macrophages, hepatocytes, fibroblasts and ilets [2021]. The present data provide the implication of CO on ER stress-mediated AKI. Considering i) the involvement of ER stress in apoptosis leading to renal tubular epithelial cell injury [10], and ii) ER stress-mediated apoptosis in response to LPS [4041], we have measured CHOP and pJNK expression as the apoptosis marker in kidney tissues. LPS-induced CHOP and pJNK expression was effectively inhibited by CORM2 under our experimental conditions, suggesting the involvement of apoptosis in ER stress-induced AKI. In addition, others markers of ER stress were reduced by CORM2 in the kidneys. However, the detailed mechanisms involved in CO-mediated suppression of ER stress have not yet been established.
From mechanical point of view, CO is membrane-permeable and directly binds to metal containing proteins such as NADPH oxidase, soluble guanylate cyclase (sGC), cytochrome P450 proteins, cytochrome c oxidase, catalase, peroxidase, and nitric oxide synthase [42]. The binding of CO to these proteins is able to confer a conformational change which can alter their biological activity [434445]. CO activates different signaling such as PI3K-AKT, PKC, PPAR-γ and MAPK which are involved in inhibition of inflammation and apoptosis [4647]. CO-mediated suppression of ER stress has been implicated in endothelial cells through activation of Nrf2/HO1 and p38 MAPK [20]. Another important signaling pathway for CO is alteration of ROS generation. Cytosolic CO inhibits the activity of NADPH oxidase and thereby, reduces superoxide production [4548]. Since ROS is associated with ER stress, CO may play an important role in inhibition of ER stress in AKI. Treatment of CORM2 not only effectively decreased oxidative stress but also significantly increased anti-oxidant genes level in LPS-treated AKI mice.
The mRNA expression of HO1, an antioxidant was increased in kidney tissues in response to LPS under our experimental conditions as shown in Fig. 4G. Decreased or deficiency of antioxidative genes such as Nrf2, HO1, and NQO1 is commonly associated with different disease pathologies including kidney injury [49505152]. On the other hand, the increased levels of Nrf2, HO1, and NQO1 are involved in adaptive responses against oxidative stress after LPS treatment in AKI mice, human monocytic cells, and cisplatin-induced AKI in rats or other kidney injury models [5354555657]. Further studies with knockdown of the molecules (i.e., Nrf2 or HO1) using siRNA are needed to strengthen the findings.
However, several questions related to our findings remain to be answered such as a) therapeutic effect of CORM2 on AKI was not investigated in the present study, b) the products of decomposition of CORM2 can generate additional effects which may contribute to the observed biological outcome, c) the optimal delivery, dosing protocol, as well as the treatment for human diseases with CO are not yet established.
In conclusion, our findings and existing evidences support that ER stress and oxidative stress inhibition by CORM2 might have protective potential in AKI. Kidney injury, ER stress, oxidative stress, and inflammation were all elevated in LPS-treated kidneys which were significantly prevented by CORM2. These results suggest that CORM2 treatment aimed at preventing ROS-mediated ER stress may hold promise to alleviate the high morbidity and mortality associated with AKI.
Figures and Tables
Fig. 1
CORM2 attenuates LPS-induced kidney injury.
To fix the optimum time required for AKI induction, mice were treated with LPS (15 mg/kg) in a time dependent manner (0, 6, 12, 24 h). (A) Kidney weight/body weight (g), (B) Plasma creatinine (mg/dl), and (C) Plasma cystatin C (ng/ml). After fixing proper AKI induction time as 18 h, we pretreated mice with CORM2 (30 mg/kg) and then treated with LPS (15 mg/kg) for 18 h. (D) Plasma creatinine (mg/dl), (E) Plasma cystatin C (ng/ml), and (F) Tubular injury marker, plasma Kim1 (pg/ml). Data are presented as means±SE of 5–8 mice/group; *p<0.05 vs. control, †p<0.05 vs. LPS.

Fig. 2
CORM2 attenuates LPS-induced inflammation in kidney.
(A) pNF-κB protein expression in the kidney was measured using western blotting. (B) Band intensities were measured using ImageJ software. (C, D) Paraffin-embedded kidney sections were stained with anti-F4/80 antibodies (1:200; original magnification: 100×; scale bar: 100 µm), and arrow marks represents the macrophage infiltration. (E) The mRNA levels of inflammation markers such as TNFα, MCP1, iNOS, and ICAM1 in kidney tissue were measured using real-time PCR. Data are presented as means±SE of 5–8 mice/group; *p<0.05 vs. control, †p<0.05 vs. LPS. (F) The mRNA levels of inflammation markers such as TNFa, MCP1, iNOS, and ICAM1 in mProx cells were measured using real-time PCR. Data are presented as means±SE, n=4; *p<0.05 vs. control, †p<0.05 vs. LPS.
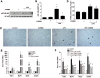
Fig. 3
CORM2 inhibits LPS-induced ER stress in kidney.
(A) pJNK protein expression was measured in the kidney using western blotting, and (B) band intensities were measured using ImageJ software. (C) CHOP protein expression was measured in the kidney using western blotting, and (D) band intensities were measured using ImageJ software. (E) ER stress markers such as sXBP1, Edem1, and GRP78 mRNA levels were measured in kidney tissue using realtime PCR. (A–E) Data are presented as means±SE of 5–8 mice/group; *p<0.05 vs. control, †p<0.05 vs. LPS. (F) The mRNA levels of ER stress markers such as sXBP1, Edem1, and GRP78 in mProx cells were measured using real-time PCR. Data are presented as means±SE, n=4; *p·0.05 vs. control, †p<0.05 vs. LPS.
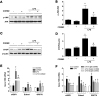
Fig. 4
CORM2 inhibits LPS-induced ER stress through suppression of ROS generation.
(A) Plasma LPO (µM), and (B) Kidney LPO (µM/mg tissue). (C) Paraffin-embedded kidney sections were stained with anti-nitrotyrosine antibodies (1:200; original magnification: 200×; scale bar: 100 µm), and arrow marks represents the accumulation of nitrotyrosine (brown color) on cytosol. (D) Frozen kidney sections were stained with DHE dye (5 µM for 10 min; original magnification: 100×; scale bar: 50 µm), and red color indicates the accumulation of ROS. The mRNA levels of antioxidant genes such as (E) Catalase, (F) Prx1, and (G) NRF2, HO1, and NQO1 in kidney tissue were measured using real-time PCR. (A–G) Data are presented as means±SE of 5–8 mice/group; *p<0.05 vs. control, †p<0.05 vs. LPS. (H) The mRNA levels of antioxidant genes such as NRF2, HO1, and NQO1 in mProx cells were measured using real-time PCR. Data are presented as means±SE, n=4; *p<0.05 vs. control, †p<0.05 vs. LPS.
ACKNOWLEDGEMENTS
This work is supported by the grants from National Research Foundation (2017R1D1A1B03028835) and Korean Research Fellowship program (2015H1D3A1062189), Republic of Korea.
References
1. Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005; 294:813–818.
2. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012; 81:442–448.

3. Lewington AJ, Cerdá J, Mehta RL. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013; 84:457–467.

4. Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015; 10:147–155.

5. Xu Y, Guo M, Jiang W, Dong H, Han Y, An XF, Zhang J. Endoplasmic reticulum stress and its effects on renal tubular cells apoptosis in ischemic acute kidney injury. Ren Fail. 2016; 38:831–837.

6. Gao X, Fu L, Xiao M, Xu C, Sun L, Zhang T, Zheng F, Mei C. The nephroprotective effect of tauroursodeoxycholic acid on ischaemia/reperfusion-induced acute kidney injury by inhibiting endoplasmic reticulum stress. Basic Clin Pharmacol Toxicol. 2012; 111:14–23.

7. Bailly-Maitre B, Fondevila C, Kaldas F, Droin N, Luciano F, Ricci JE, Croxton R, Krajewska M, Zapata JM, Kupiec-Weglinski JW, Farmer D, Reed JC. Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2006; 103:2809–2814.

8. Li X, Hassoun HT, Santora R, Rabb H. Organ crosstalk: the role of the kidney. Curr Opin Crit Care. 2009; 15:481–487.

9. Mittwede PN, Xiang L, Lu S, Clemmer JS, Hester RL. Oxidative stress contributes to orthopedic trauma-induced acute kidney injury in obese rats. Am J Physiol Renal Physiol. 2015; 308:F157–F163.

10. Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z. Regulated cell death in AKI. J Am Soc Nephrol. 2014; 25:2689–2701.

11. Gao L, Wu WF, Dong L, Ren GL, Li HD, Yang Q, Li XF, Xu T, Li Z, Wu BM, Ma TT, Huang C, Huang Y, Zhang L, Lv X, Li J, Meng XM. Protocatechuic aldehyde attenuates cisplatin-induced acute kidney injury by suppressing nox-mediated oxidative stress and renal inflammation. Front Pharmacol. 2016; 7:479.

12. Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002; 415:92–96.

13. Kim S, Joe Y, Kim HJ, Kim YS, Jeong SO, Pae HO, Ryter SW, Surh YJ, Chung HT. Endoplasmic reticulum stress-induced IRE1α activation mediates cross-talk of GSK-3β and XBP-1 to regulate inflammatory cytokine production. J Immunol. 2015; 194:4498–4506.

14. Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000; 287:664–666.

15. Lin H, Liu XB, Yu JJ, Hua F, Hu ZW. Antioxidant N-acetylcysteine attenuates hepatocarcinogenesis by inhibiting ROS/ER stress in TLR2 deficient mouse. PLoS One. 2013; 8:e74130.

16. Wang Q, Wang H, Jia Y, Pan H, Ding H. Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. Cancer Chemother Pharmacol. 2017; 79:1031–1041.

17. Nakahira K, Choi AM. Carbon monoxide in the treatment of sepsis. Am J Physiol Lung Cell Mol Physiol. 2015; 309:L1387–L1393.

18. Ryter SW, Morse D, Choi AM. Carbon monoxide: to boldly go where NO has gone before. Sci STKE. 2004; 2004:RE6.

19. Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999; 276:L688–L694.
20. Kim KM, Pae HO, Zheng M, Park R, Kim YM, Chung HT. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res. 2007; 101:919–927.

21. Kim DS, Song L, Wang J, Wu H, Gou W, Cui W, Kim JS, Wang H. Carbon monoxide inhibits islet apoptosis via induction of autophagy. Antioxid Redox Signal. 2018; 28:1309–1322.

22. Wang L, Lee JY, Kwak JH, He Y, Kim SI, Choi ME. Protective effects of low-dose carbon monoxide against renal fibrosis induced by unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2008; 294:F508–F517.

23. Shiohira S, Yoshida T, Shirota S, Tsuchiya K, Nitta K. Protective effect of carbon monoxide donor compounds in endotoxin-induced acute renal failure. Am J Nephrol. 2007; 27:441–446.

24. Wang P, Huang J, Li Y, Chang R, Wu H, Lin J, Huang Z. Exogenous carbon monoxide decreases sepsis-induced acute kidney injury and inhibits NLRP3 inflammasome activation in rats. Int J Mol Sci. 2015; 16:20595–20608.
25. Tayem Y, Johnson TR, Mann BE, Green CJ, Motterlini R. Protection against cisplatin-induced nephrotoxicity by a carbon monoxide-releasing molecule. Am J Physiol Renal Physiol. 2006; 290:F789–F794.

26. Neto JS, Nakao A, Kimizuka K, Romanosky AJ, Stolz DB, Uchiyama T, Nalesnik MA, Otterbein LE, Murase N. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am J Physiol Renal Physiol. 2004; 287:F979–F989.

27. Vera T, Henegar JR, Drummond HA, Rimoldi JM, Stec DE. Protective effect of carbon monoxide-releasing compounds in ischemia-induced acute renal failure. J Am Soc Nephrol. 2005; 16:950–958.

28. Zhang X, Shan P, Otterbein LE, Alam J, Flavell RA, Davis RJ, Choi AM, Lee PJ. Carbon monoxide inhibition of apoptosis during ischemia-reperfusion lung injury is dependent on the p38 mitogen-activated protein kinase pathway and involves caspase 3. J Biol Chem. 2003; 278:1248–1258.

29. Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980; 29:189–201.

30. Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000; 13:110–116.

31. Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009; 119:2868–2878.

32. Tiwari MM, Brock RW, Megyesi JK, Kaushal GP, Mayeux PR. Disruption of renal peritubular blood flow in lipopolysaccharide-induced renal failure: role of nitric oxide and caspases. Am J Physiol Renal Physiol. 2005; 289:F1324–F1332.

33. Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1 release in vitro and improve survival of mice in LPS- and cecal ligation and puncture-induced sepsis model in vivo. Mol Pharmacol. 2009; 76:173–182.

34. Ha H, Yu MR, Kim KH. Melatonin and taurine reduce early glomerulopathy in diabetic rats. Free Radic Biol Med. 1999; 26:944–950.

35. Park JH, Ha H. Short-term treatment of daumone improves hepatic inflammation in aged mice. Korean J Physiol Pharmacol. 2015; 19:269–274.

36. Sohn M, Kim K, Uddin MJ, Lee G, Hwang I, Kang H, Kim H, Lee JH, Ha H. Delayed treatment with fenofibrate protects against high-fat diet-induced kidney injury in mice: the possible role of AMPK autophagy. Am J Physiol Renal Physiol. 2017; 312:F323–F334.

37. Sancho-Martínez SM, López-Novoa JM, López-Hernández FJ. Pathophysiological role of different tubular epithelial cell death modes in acute kidney injury. Clin Kidney J. 2015; 8:548–559.

38. Maeshima A, Takahashi S, Nakasatomi M, Nojima Y. Diverse cell populations involved in regeneration of renal tubular epithelium following acute kidney injury. Stem Cells Int. 2015; DOI: 10.1155/2015/964849.

39. Abe T, Yazawa K, Fujino M, Imamura R, Hatayama N, Kakuta Y, Tsutahara K, Okumi M, Ichimaru N, Kaimori JY, Isaka Y, Seki K, Takahara S, Li XK, Nonomura N. High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab Invest. 2017; 97:468–477.

40. Kim HJ, Jeong JS, Kim SR, Park SY, Chae HJ, Lee YC. Inhibition of endoplasmic reticulum stress alleviates lipopolysaccharide-induced lung inflammation through modulation of NF-κB/HIF-1α signaling pathway. Sci Rep. 2013; 3:1142.

41. Zhang H, Zhang W, Jiao F, Li X, Zhang H, Wang L, Gong Z. The nephroprotective effect of MS-275 on lipopolysaccharide (LPS)-induced acute kidney injury by inhibiting reactive oxygen species (ROS)-oxidative stress and endoplasmic reticulum stress. Med Sci Monit. 2018; 24:2620–2630.

42. Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006; 46:411–449.

43. Roberts GP, Youn H, Kerby RL. CO-sensing mechanisms. Microbiol Mol Biol Rev. 2004; 68:453–473. table of contents.

44. Taillé C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005; 280:25350–25360.
45. Matsumoto H, Ishikawa K, Itabe H, Maruyama Y. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction. Mol Cell Biochem. 2006; 291:21–28.

46. Dulak J, Loboda A, Jozkowicz A. Effect of heme oxygenase-1 on vascular function and disease. Curr Opin Lipidol. 2008; 19:505–512.

47. Csongradi E, Juncos LA, Drummond HA, Vera T, Stec DE. Role of carbon monoxide in kidney function: is a little carbon monoxide good for the kidney? Curr Pharm Biotechnol. 2012; 13:819–826.

48. Srisook K, Han SS, Choi HS, Li MH, Ueda H, Kim C, Cha YN. CO from enhanced HO activity or from CORM-2 inhibits both O2- and NO production and downregulates HO-1 expression in LPS-stimulated macrophages. Biochem Pharmacol. 2006; 71:307–318.

49. Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol. 2000; 11:965–973.
50. Cho HY, Reddy SP, Kleeberger SR. Nrf2 defends the lung from oxidative stress. Antioxid Redox Signal. 2006; 8:76–87.

51. Liu M, Grigoryev DN, Crow MT, Haas M, Yamamoto M, Reddy SP, Rabb H. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009; 76:277–285.

52. Kim TW, Kim YJ, Kim HT, Park SR, Lee MY, Park YD, Lee CH, Jung JY. NQO1 deficiency leads enhanced autophagy in cisplatin-induced acute kidney injury through the AMPK/TSC2/mTOR signaling pathway. Antioxid Redox Signal. 2016; 24:867–883.

53. Lee JW, Kwon JH, Lim MS, Lee HJ, Kim SS, Lim SY, Chun W. 3,4,5-Trihydroxycinnamic acid increases heme-oxygenase-1 (HO-1) and decreases macrophage infiltration in LPS-induced septic kidney. Mol Cell Biochem. 2014; 397:109–116.

54. Rushworth SA, MacEwan DJ, O'Connell MA. Lipopolysaccharide-induced expression of NAD(P)H:quinone oxidoreductase 1 and heme oxygenase-1 protects against excessive inflammatory responses in human monocytes. J Immunol. 2008; 181:6730–6737.

55. Rushworth SA, Chen XL, Mackman N, Ogborne RM, O’Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J Immunol. 2005; 175:4408–4415.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download