This article has been
cited by other articles in ScienceCentral.
Transcatheter aortic valve replacement (TAVR) in aortic regurgitation (AR) has not been well established, because the absence of an anchoring point such as calcification at the annulus and leaflets is known to be an obstacle to the procedure.
1)
A 79-year-old female patient had severe AR after a Comprehensive Aortic Root and VAlve Repair (CARVAR) operation presenting with worsening dyspnea (
Supplementary Video 1). CARVAR operation is a valve sparing surgical technique for aortic diseases, consisting of annular and sinotubular junction (STJ) reduction with leaflet correction.
2) For reduction and correction of aortic structures, specially designed outer strip ring is used in CARVAR procedure. However, this novel technique which had been criticized for safety issues comparing with the conventional valve replacement surgery was banned in South Korea since 2013.
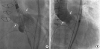
3) Due to high surgical risk (Society of Thoracic Surgeon's predicted risk of mortality score 14.756%) and frailty of the patient (clinical frailty scale of 6), heart team decided to proceed with the TAVR procedure. In preprocedural device selection, the perimeter of the annulus was 71.9 mm and the perimeter driven diameter was 22.9 mm in multi-slice computed tomography (CT) images. The diameter of STJ with outer ring was measured as 27 mm. We thought that 2 possible anchoring points were annulus and STJ with outer ring. Therefore, a 26 or 29 mm transcatheter heart valve seemed to be possible according to the conventional annular valve sizing because perimeter or diameter was on the gray zone. Considering TAVI for AR with no calcification at annulus, larger one would be better. In addition, considering the possibility that the upper part of the transcatheter heart valve is anchored to the 27 mm-sized CARVAR ring located in the STJ instead of annulus, a transcatheter heart valve of 29 or 31 mm is considered to be appropriate. Finally, we chose a 29 mm Evolut™ R valve instead of a larger sized transcatheter heart valve due to possible risk of device deformation by outer strip ring. Despite the absence of any calcification at the annulus and leaflets (
Figure 1), a 29 mm CoreValve
® Evolut™ R valve (Medtronic, Minneapolis, MN, USA) was successfully implanted at a slightly lower location than the usual position utilizing an outer ring used in CARVAR surgery as the landing zone (
Figure 2,
Supplementary Videos 2 and
3). Upper part between outflow and waist of 29 mm Evolut™ R valve was seated at the outer strip ring.
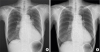
After TAVR procedure, the patient's dyspnea improved markedly. Echocardiography showed well-functioning transcatheter heart valve without any definite evidence of central AR or paravalvular leakage (
Supplementary Video 4). N-terminal brain natriuretic peptides reduced from 10,027 to 2,708 pg/mL and cardiomegaly decreased (
Figure 3).
These images highlight the importance of adequate anchoring points when performing TAVR for AR.
4)5)





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download