1. Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Europace. 2009; 11:771–817. PMID:
19443434.
2. Svenson RH, Littmann L, Gallagher JJ, et al. Termination of ventricular tachycardia with epicardial laser photocoagulation: a clinical comparison with patients undergoing successful endocardial photocoagulation alone. J Am Coll Cardiol. 1990; 15:163–170. PMID:
2295728.
3. Downar E, Parson ID, Mickleborough LL, Cameron DA, Yao LC, Waxman MB. On-line epicardial mapping of intraoperative ventricular arrhythmias: initial clinical experience. J Am Coll Cardiol. 1984; 4:703–714. PMID:
6481011.
4. Kaltenbrunner W, Cardinal R, Dubuc M, et al. Epicardial and endocardial mapping of ventricular tachycardia in patients with myocardial infarction. Is the origin of the tachycardia always subendocardially localized? Circulation. 1991; 84:1058–1071. PMID:
1884439.
5. Littmann L, Svenson RH, Gallagher JJ, et al. Functional role of the epicardium in postinfarction ventricular tachycardia. Observations derived from computerized epicardial activation mapping, entrainment, and epicardial laser photoablation. Circulation. 1991; 83:1577–1591. PMID:
2022017.
6. Blanchard SM, Walcott GP, Wharton JM, Ideker RE. Why is catheter ablation less successful than surgery for treating ventricular tachycardia that results from coronary artery disease? Pacing Clin Electrophysiol. 1994; 17:2315–2335. PMID:
7885941.
7. Morady F, Harvey M, Kalbfleisch SJ, el-Atassi R, Calkins H, Langberg JJ. Radiofrequency catheter ablation of ventricular tachycardia in patients with coronary artery disease. Circulation. 1993; 87:363–372. PMID:
8425285.
8. Sosa E, Scanavacca M, d'Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996; 7:531–536. PMID:
8743758.
9. Sosa E, Scanavacca M, d'Avila A, et al. Endocardial and epicardial ablation guided by nonsurgical transthoracic epicardial mapping to treat recurrent ventricular tachycardia. J Cardiovasc Electrophysiol. 1998; 9:229–239. PMID:
9580377.
10. Sacher F, Roberts-Thomson K, Maury P, et al. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010; 55:2366–2372. PMID:
20488308.
11. Gonska BD, Cao K, Schaumann A, Dorszewski A, von zur Mühlen F, Kreuzer H. Catheter ablation of ventricular tachycardia in 136 patients with coronary artery disease: results and long-term follow-up. J Am Coll Cardiol. 1994; 24:1506–1514. PMID:
7930283.
12. Stevenson WG, Friedman PL, Kocovic D, Sager PT, Saxon LA, Pavri B. Radiofrequency catheter ablation of ventricular tachycardia after myocardial infarction. Circulation. 1998; 98:308–314. PMID:
9711935.
13. Stevenson WG, Wilber DJ, Natale A, et al. Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction: the multicenter thermocool ventricular tachycardia ablation trial. Circulation. 2008; 118:2773–2782. PMID:
19064682.
14. Sacher F, Tedrow UB, Field ME, et al. Ventricular tachycardia ablation: evolution of patients and procedures over 8 years. Circ Arrhythm Electrophysiol. 2008; 1:153–161. PMID:
19808409.
15. Soejima K, Stevenson WG, Sapp JL, Selwyn AP, Couper G, Epstein LM. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004; 43:1834–1842. PMID:
15145109.
16. Berruezo A, Mont L, Nava S, Chueca E, Bartholomay E, Brugada J. Electrocardiographic recognition of the epicardial origin of ventricular tachycardias. Circulation. 2004; 109:1842–1847. PMID:
15078793.
17. Boyle NG, Shivkumar K. Epicardial interventions in electrophysiology. Circulation. 2012; 126:1752–1769. PMID:
23027811.
18. Martinek M, Stevenson WG, Inada K, Tokuda M, Tedrow UB. QRS characteristics fail to reliably identify ventricular tachycardias that require epicardial ablation in ischemic heart disease. J Cardiovasc Electrophysiol. 2012; 23:188–193. PMID:
21955120.
19. Bazan V, Gerstenfeld EP, Garcia FC, et al. Site-specific twelve-lead ECG features to identify an epicardial origin for left ventricular tachycardia in the absence of myocardial infarction. Heart Rhythm. 2007; 4:1403–1410. PMID:
17954399.
20. Bazan V, Bala R, Garcia FC, et al. Twelve-lead ECG features to identify ventricular tachycardia arising from the epicardial right ventricle. Heart Rhythm. 2006; 3:1132–1139. PMID:
17018339.
21. Vallès E, Bazan V, Marchlinski FE. ECG criteria to identify epicardial ventricular tachycardia in nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2010; 3:63–71. PMID:
20008307.
22. Daniels DV, Lu YY, Morton JB, et al. Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of Valsalva: electrophysiological characteristics, catheter ablation, and identification from the 12-lead electrocardiogram. Circulation. 2006; 113:1659–1666. PMID:
16567566.
23. Wijnmaalen AP, van der Geest RJ, van Huls van Taxis CF, et al. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration. Eur Heart J. 2011; 32:104–114. PMID:
20864488.
24. Andreu D, Berruezo A, Ortiz-Pérez JT, et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2011; 4:674–683. PMID:
21880674.
25. Bogun FM, Desjardins B, Good E, et al. Delayed-enhanced magnetic resonance imaging in nonischemic cardiomyopathy: utility for identifying the ventricular arrhythmia substrate. J Am Coll Cardiol. 2009; 53:1138–1145. PMID:
19324259.
26. Bala R, Ren JF, Hutchinson MD, et al. Assessing epicardial substrate using intracardiac echocardiography during VT ablation. Circ Arrhythm Electrophysiol. 2011; 4:667–673. PMID:
21880675.
27. Tung R, Michowitz Y, Yu R, et al. Epicardial ablation of ventricular tachycardia: an institutional experience of safety and efficacy. Heart Rhythm. 2013; 10:490–498. PMID:
23246598.
28. Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009; 54:799–808. PMID:
19695457.
29. Della Bella P, Brugada J, Zeppenfeld K, et al. Epicardial ablation for ventricular tachycardia: a European multicenter study. Circ Arrhythm Electrophysiol. 2011; 4:653–659. PMID:
21841191.
30. Scanavacca M. Epicardial ablation for ventricular tachycardia in chronic Chagas heart disease. Arq Bras Cardiol. 2014; 102:524–528. PMID:
25004413.
31. Henz BD, do Nascimento TA, Dietrich CO, et al. Simultaneous epicardial and endocardial substrate mapping and radiofrequency catheter ablation as first-line treatment for ventricular tachycardia and frequent ICD shocks in chronic chagasic cardiomyopathy. J Interv Card Electrophysiol. 2009; 26:195–205. PMID:
19757003.
32. Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996; 94:983–991. PMID:
8790036.
33. Wichter T, Paul TM, Eckardt L, et al. Arrhythmogenic right ventricular cardiomyopathy. Antiarrhythmic drugs, catheter ablation, or ICD? Herz. 2005; 30:91–101. PMID:
15875097.
34. Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009; 120:366–375. PMID:
19620503.
35. Berruezo A, Fernández-Armenta J, Mont L, et al. Combined endocardial and epicardial catheter ablation in arrhythmogenic right ventricular dysplasia incorporating scar dechanneling technique. Circ Arrhythm Electrophysiol. 2012; 5:111–121. PMID:
22205683.
36. Bai R, Di Biase L, Shivkumar K, et al. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol. 2011; 4:478–485. PMID:
21665983.
37. Dello Russo A, Casella M, Pieroni M, et al. Drug-refractory ventricular tachycardias after myocarditis: endocardial and epicardial radiofrequency catheter ablation. Circ Arrhythm Electrophysiol. 2012; 5:492–498. PMID:
22294614.
38. Koplan BA, Soejima K, Baughman K, Epstein LM, Stevenson WG. Refractory ventricular tachycardia secondary to cardiac sarcoid: electrophysiologic characteristics, mapping, and ablation. Heart Rhythm. 2006; 3:924–929. PMID:
16876741.
39. Papageorgiou N, Providência R, Bronis K, et al. Catheter ablation for ventricular tachycardia in patients with cardiac sarcoidosis: a systematic review. Europace. 2018; 20:682–691. PMID:
28444174.
40. Fernandes GC, Fernandes A, Cardoso R, et al. Ablation strategies for the management of symptomatic Brugada syndrome: a systematic review. Heart Rhythm. 2018; [Epub ahead of print].
41. Pisani CF, Lara S, Scanavacca M. Epicardial ablation for cardiac arrhythmias: techniques, indications and results. Curr Opin Cardiol. 2014; 29:59–67. PMID:
24270396.
42. Kumar S, Bazaz R, Barbhaiya CR, et al. “Needle-in-needle” epicardial access: Preliminary observations with a modified technique for facilitating epicardial interventional procedures. Heart Rhythm. 2015; 12:1691–1697. PMID:
25828599.
43. Soejima K, Couper G, Cooper JM, Sapp JL, Epstein LM, Stevenson WG. Subxiphoid surgical approach for epicardial catheter-based mapping and ablation in patients with prior cardiac surgery or difficult pericardial access. Circulation. 2004; 110:1197–1201. PMID:
15337702.
44. Koruth JS, Aryana A, Dukkipati SR, et al. Unusual complications of percutaneous epicardial access and epicardial mapping and ablation of cardiac arrhythmias. Circ Arrhythm Electrophysiol. 2011; 4:882–888. PMID:
22007036.
45. d'Avila A, Houghtaling C, Gutierrez P, et al. Catheter ablation of ventricular epicardial tissue: a comparison of standard and cooled-tip radiofrequency energy. Circulation. 2004; 109:2363–2369. PMID:
15096448.
46. Aryana A, O'Neill PG, Pujara DK, et al. Impact of irrigation flow rate and intrapericardial fluid on cooled-tip epicardial radiofrequency ablation. Heart Rhythm. 2016; 13:1602–1611. PMID:
27165695.
47. Sacher F, Wright M, Derval N, et al. Endocardial versus epicardial ventricular radiofrequency ablation: utility of in vivo contact force assessment. Circ Arrhythm Electrophysiol. 2013; 6:144–150. PMID:
23392586.
48. Wong MC, Edwards G, Spence SJ, et al. Characterization of catheter-tissue contact force during epicardial radiofrequency ablation in an ovine model. Circ Arrhythm Electrophysiol. 2013; 6:1222–1228. PMID:
24134869.
49. Jesel L, Sacher F, Komatsu Y, et al. Characterization of contact force during endocardial and epicardial ventricular mapping. Circ Arrhythm Electrophysiol. 2014; 7:1168–1173. PMID:
25258362.
50. d'Avila A, Aryana A, Thiagalingam A, et al. Focal and linear endocardial and epicardial catheter-based cryoablation of normal and infarcted ventricular tissue. Pacing Clin Electrophysiol. 2008; 31:1322–1331. PMID:
18811814.
51. Lustgarten DL, Bell S, Hardin N, Calame J, Spector PS. Safety and efficacy of epicardial cryoablation in a canine model. Heart Rhythm. 2005; 2:82–90. PMID:
15851270.
52. d'Avila A. Epicardial catheter ablation of ventricular tachycardia. Heart Rhythm. 2008; 5:S73–5. PMID:
18456208.
53. Saba MM, Akella J, Gammie J, et al. The influence of fat thickness on the human epicardial bipolar electrogram characteristics: measurements on patients undergoing open-heart surgery. Europace. 2009; 11:949–953. PMID:
19546189.
54. Abbara S, Desai JC, Cury RC, Butler J, Nieman K, Reddy V. Mapping epicardial fat with multi-detector computed tomography to facilitate percutaneous transepicardial arrhythmia ablation. Eur J Radiol. 2006; 57:417–422. PMID:
16434161.
55. Silaghi A, Piercecchi-Marti MD, Grino M, et al. Epicardial adipose tissue extent: relationship with age, body fat distribution, and coronaropathy. Obesity (Silver Spring). 2008; 16:2424–2430. PMID:
18719675.
56. Ahn SG, Lim HS, Joe DY, et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008; 94:e7. PMID:
17923467.
57. Tung R, Nakahara S, Ramirez R, Lai C, Fishbein MC, Shivkumar K. Distinguishing epicardial fat from scar: analysis of electrograms using high-density electroanatomic mapping in a novel porcine infarct model. Heart Rhythm. 2010; 7:389–395. PMID:
20185114.
58. van Huls van Taxis CF, Wijnmaalen AP, Piers SR, van der Geest RJ, Schalij MJ, Zeppenfeld K. Real-time integration of MDCT-derived coronary anatomy and epicardial fat: impact on epicardial electroanatomic mapping and ablation for ventricular arrhythmias. JACC Cardiovasc Imaging. 2013; 6:42–52. PMID:
23328560.
59. Hong KN, Russo MJ, Liberman EA, et al. Effect of epicardial fat on ablation performance: a three-energy source comparison. J Card Surg. 2007; 22:521–524. PMID:
18039220.
60. Desjardins B, Morady F, Bogun F. Effect of epicardial fat on electroanatomical mapping and epicardial catheter ablation. J Am Coll Cardiol. 2010; 56:1320–1327. PMID:
20888522.
61. Sosa E, Scanavacca M. Epicardial mapping and ablation techniques to control ventricular tachycardia. J Cardiovasc Electrophysiol. 2005; 16:449–452. PMID:
15828893.
62. Javaheri A, Glassberg HL, Acker MA, Callans DJ, Goldberg LR. Constrictive pericarditis presenting as a late complication of epicardial ventricular tachycardia ablation. Circ Heart Fail. 2012; 5:e22–e23. PMID:
22438524.
63. d'Avila A, Neuzil P, Thiagalingam A, et al. Experimental efficacy of pericardial instillation of anti-inflammatory agents during percutaneous epicardial catheter ablation to prevent postprocedure pericarditis. J Cardiovasc Electrophysiol. 2007; 18:1178–1183. PMID:
17887979.
64. Viles-Gonzalez JF, de Castro Miranda R, Scanavacca M, Sosa E, d'Avila A. Acute and chronic effects of epicardial radiofrequency applications delivered on epicardial coronary arteries. Circ Arrhythm Electrophysiol. 2011; 4:526–531. PMID:
21685298.
65. Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm. 2009; 6:886–933. PMID:
19467519.
66. d'Avila A, Gutierrez P, Scanavacca M, et al. Effects of radiofrequency pulses delivered in the vicinity of the coronary arteries: implications for nonsurgical transthoracic epicardial catheter ablation to treat ventricular tachycardia. Pacing Clin Electrophysiol. 2002; 25:1488–1495. PMID:
12418747.
67. Bai R, Patel D, Di Biase L, et al. Phrenic nerve injury after catheter ablation: should we worry about this complication? J Cardiovasc Electrophysiol. 2006; 17:944–948. PMID:
16800858.
68. Di Biase L, Burkhardt JD, Pelargonio G, et al. Prevention of phrenic nerve injury during epicardial ablation: comparison of methods for separating the phrenic nerve from the epicardial surface. Heart Rhythm. 2009; 6:957–961. PMID:
19560084.
69. Mathuria N, Buch E, Shivkumar K. Pleuropericardial fistula formation after prior epicardial catheter ablation for ventricular tachycardia. Circ Arrhythm Electrophysiol. 2012; 5:e18–e19. PMID:
22334435.
70. Tschabrunn CM, Haqqani HM, Zado ES, Marchlinski FE. Repeat percutaneous epicardial mapping and ablation of ventricular tachycardia: safety and outcome. J Cardiovasc Electrophysiol. 2012; 23:744–749. PMID:
22353308.
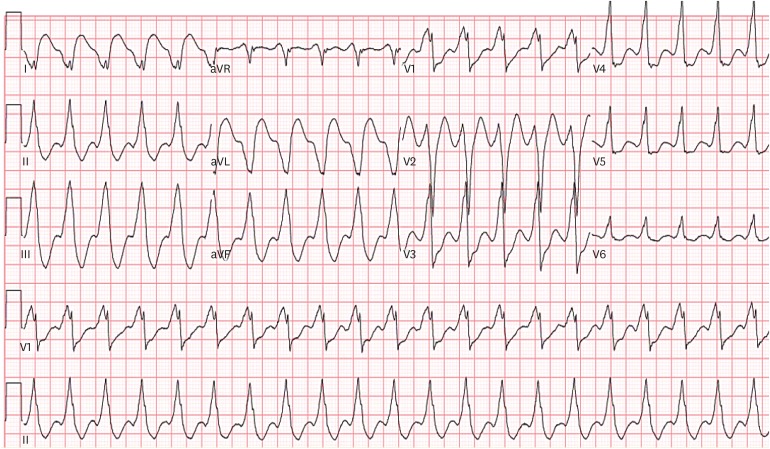
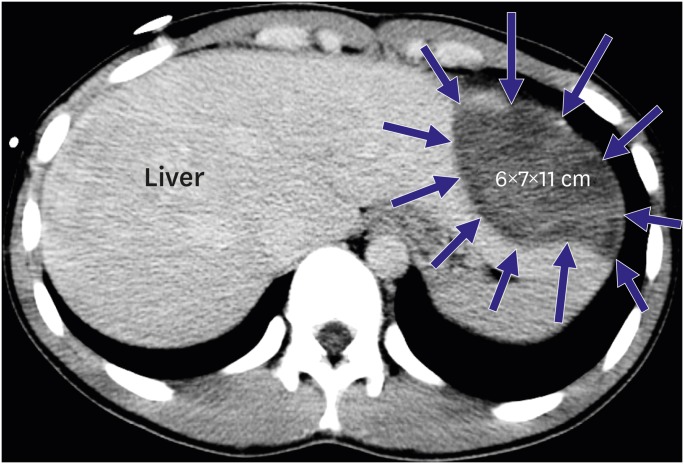
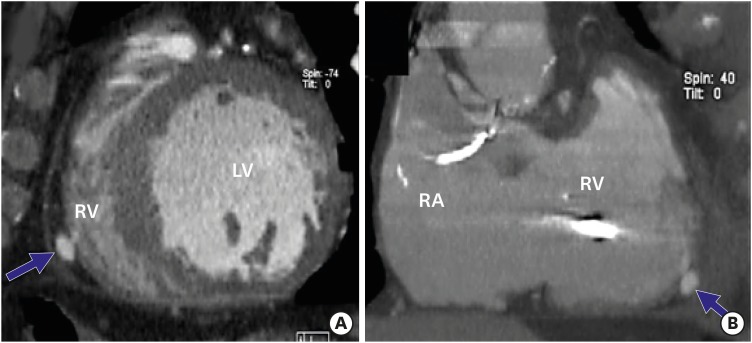




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download