1. Prinzmetal M, Kennamer R, Merliss R, Wada T, Bor N. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am J Med. 1959; 27:375–388.
2. Oliva PB, Potts DE, Pluss RG. Coronary arterial spasm in Prinzmetal angina. Documentation by coronary arteriography. N Engl J Med. 1973; 288:745–751.
3. Maseri A. Louis F. Bishop lecture. Role of coronary artery spasm in symptomatic and silent myocardial ischemia. J Am Coll Cardiol. 1987; 9:249–262.
4. Heupler FA Jr, Proudfit WL, Razavi M, Shirey EK, Greenstreet R, Sheldon WC. Ergonovine maleate provocative test for coronary arterial spasm. Am J Cardiol. 1978; 41:631–640.

5. Yasue H, Horio Y, Nakamura N, et al. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986; 74:955–963.

6. Hackett D, Larkin S, Chierchia S, Davies G, Kaski JC, Maseri A. Induction of coronary artery spasm by a direct local action of ergonovine. Circulation. 1987; 75:577–582.

7. Park SW, Park SJ, Kim JJ, Song JK, Seong IW, Lee JK. A comparative study of acetylcholine and ergonovine provocative test in patients with chest pain syndrome with normal or near normal coronary arteriograms. Korean Circ J. 1991; 21:842–848.

8. Zaya M, Mehta PK, Merz NB. Provocative testing for coronary reactivity and spasm. J Am Coll Cardiol. 2014; 63:103–109.
9. MacAlpin RN. Some observations on and controversies about coronary arterial spasm. Int J Cardiol. 2015; 181:389–398.

10. Beltrame JF, Crea F, Kaski JC, et al. International standardization of diagnostic criteria for vasospastic angina. Eur Heart J. 2017; 38:2565–2568.

11. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012; 60:e44–164.
12. Anderson JL, Adams CD, Antman EM, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013; 61:e179–347.
13. JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014; 78:2779–2801.
14. Yasue H, Nagao M, Omote S, Takizawa A, Miwa K, Tanaka S. Coronary arterial spasm and Prinzmetal’s variant form of angina induced by hyperventilation and Tris-buffer infusion. Circulation. 1978; 58:56–62.

15. Waters DD, Szlachcic J, Bonan R, Miller DD, Dauwe F, Theroux P. Comparative sensitivity of exercise, cold pressor and ergonovine testing in provoking attacks of variant angina in patients with active disease. Circulation. 1983; 67:310–315.

16. Bertrand ME, LaBlanche JM, Tilmant PY, et al. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary arteriography. Circulation. 1982; 65:1299–1306.

17. Takagi Y, Yasuda S, Takahashi J, et al. Clinical implications of provocation tests for coronary artery spasm: safety, arrhythmic complications, and prognostic impact: multicentre registry study of the Japanese Coronary Spasm Association. Eur Heart J. 2013; 34:258–267.

18. Song JK, Park SW, Kim JJ, et al. Values of intravenous ergonovine test with two-dimensional echocardiography for diagnosis of coronary artery spasm. J Am Soc Echocardiogr. 1994; 7:607–615.

19. Song JK, Lee SJ, Kang DH, et al. Ergonovine echocardiography as a screening test for diagnosis of vasospastic angina before coronary angiography. J Am Coll Cardiol. 1996; 27:1156–1161.

20. Djordjevic-Dikic A, Varga A, Rodriguez O, et al. Safety of ergotamine-ergic pharmacologic stress echocardiography for vasospasm testing in the echo lab: 14 year experience on 478 tests in 464 patients. Cardiologia. 1999; 44:901–906.
21. Song JK, Park SW, Kang DH, et al. Safety and clinical impact of ergonovine stress echocardiography for diagnosis of coronary vasospasm. J Am Coll Cardiol. 2000; 35:1850–1856.

22. Buxton A, Goldberg S, Hirshfeld JW, et al. Refractory ergonovine-induced coronary vasospasm: importance of intracoronary nitroglycerin. Am J Cardiol. 1980; 46:329–334.

23. Nesto RW, Kowalchuck GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol. 1987; 59:23C–30C.

24. Yasue H, Takizawa A, Nagao M, et al. Long-term prognosis for patients with variant angina and influential factors. Circulation. 1988; 78:1–9.

25. Ahn JM, Lee KH, Yoo SY, et al. Prognosis of variant angina manifesting as aborted sudden cardiac death. J Am Coll Cardiol. 2016; 68:137–145.
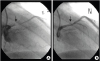

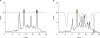
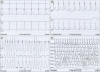
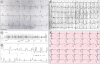






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download