Abstract
Background
The incidence of cardiovascular and neurovascular diseases has been increasing with the aging of the population, and antiplatelet drugs (APDs) are more frequently used than in the past. With the average age of spinal surgery patients also increasing, there has been a great concern on the adverse effects of APD on spine surgery. To our knowledge, though there have been many studies on this issue, their results are conflicting. In this study, we aimed to determine the influence of APDs on spine surgery in terms of intraoperative bleeding and postoperative spinal epidural hematoma complication.
Methods
Patients who underwent posterior thoracolumbar decompression and instrumentation at our institution were reviewed. There were 34 APD takers (APDT group). Seventy-nine non-APD takers (NAPDT group) were selected as a control group in consideration of demographic and surgical factors. There were two primary endpoints of this study: the amount of bleeding per 10 minutes and cauda equina compression by epidural hematoma measured at the cross-sectional area of the thecal sac in the maximal compression site on the axial T2 magnetic resonance imaging scans taken on day 7.
Results
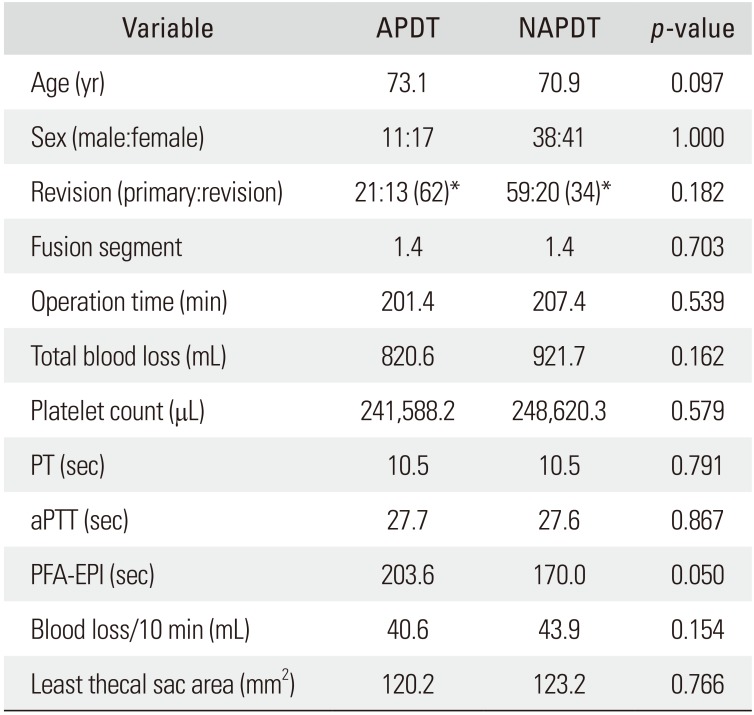
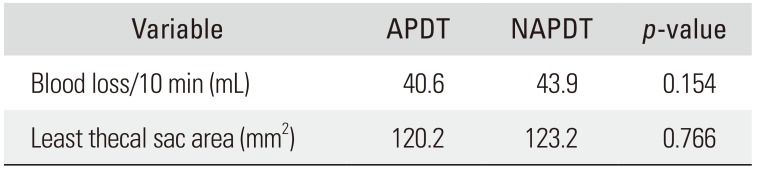
Both groups were homogeneous regarding age and sex (demographic factors), the number of fused segments, operation time, and primary/revision operation (surgical factors), and the number of platelets, prothrombin time, and activated partial thromboplastin time (coagulation-related factors). However, the platelet function analysis-epinephrine was delayed in the APDT group than in the NAPDT group (203.6 seconds vs. 170.0 seconds, p = 0.050). Intraoperative bleeding per 10 minutes was 40.6 ± 12.8 mL in the APDT group and 43.9 ± 9.9 mL in the NAPDT group, showing no significant difference between the two groups (p = 0.154). The cross-sectional area of the thecal sac at the maximal compression site by epidural hematoma was 120.2 ± 48.2 mm2 in the APDT group and 123.2 ± 50.4 mm2 in the NAPDT group, showing no significant difference between the two groups (p = 0.766).
The prevalence of cardiovascular and neurovascular diseases has been increasing with the aging of the population. The use of antiplatelet drugs (APDs) contributes to prevention of such diseases; at the same time, it increases bleeding tendency in invasive procedures and operations. Discontinuance of APDs before noncardiac surgery, however, is a major cause of concern among surgeons because it could bring about more disastrous consequences. APD cessation in patients with a history of coronary artery intervention has been associated with a 5- to 10-fold increase in the mortality rate due to acute myocardial infarction.1) Though there are many studies reporting increased incidences of bleeding-related complications in APD users in various surgeries,234) there have been few studies focused on spine surgery. In a spine surgery carried out without APD interruption, increased bleeding may complicate the procedure and lead to postoperative spinal epidural hematoma (POSEH) resulting in neurological deficit. Though APDs increase bleeding tendency, it is worth noting that intraoperative bleeding may also be influenced by assistance of the anesthesiologist and surgeon's efforts to control bleeding. While many studies have demonstrated that APDs increase the risk of spontaneous and postinvasive procedural cases spinal epidural hematoma,567) there has been no general consensus on postoperative hematoms.89) In this study, we attempted to verify the influence of APD on spine surgery based on the assessment of the amount of bleeding per unit time and development of POSEH.
The data was collected prospectively and analyzed retrospectively. All thoracolumbar decompression and instrumented fusions performed through a posterior approach at our institution by the senior author (DKA) were reviewed. To improve the homogeneity of the data, only instrumented surgeries were included, and minimally invasive surgeries were excluded. There were 449 cases that met the inclusion criteria. Among them, 284 cases (63%) consented to take postoperative magnetic resonance imaging (MRI). Thirty-four out of 284 cases discontinued APD therapy less than 3 days before surgery and 250 cases did not take or discontinued APD therapy more than 4 days before spine surgery. Among the latter 250 cases, 79 cases were selected by stratified sampling in terms of age and sex. Finally, 34 cases classified as APD takers (APDT group) and 79 cases as non-APD takers (NAPDT group) were included in the study (Fig. 1). The reasons for APD use (Table 1) and specific APD names are also tabulated (Table 2). Patients began to take the same kind of APD they had been on before surgery after nil per os was lifted. All operations were performed through a conventional posterior approach. Two lines of suction drain were placed, and each line was connected to a respective 120 ± 30 mmHg vacuum bag (Ez-VAC; eG Medisys, Goyang, Korea). All drains were removed on postoperative day 3 irrespective of the drained blood amount. The homogeneity of the two groups were analyzed using demographic factors (age and sex), surgical factors (number of fusion segments, operation time, primary/revision surgery), and coagulation-related factors (number of platelets, prothrombin time, activated partial thromboplastin time, and platelet function analysis-epinephrine [PFA-EPI]). Postoperative MRI was performed on postoperative day 7. On the T2-weighted axial image showing the narrowest thecal sac area by compression of epidural hematoma, the cross-sectional area of the thecal sac was measured by region of interest technique. INFINITT PACS (INFINITT Healthcare System, Seoul, Korea) system was used for the measurement (Fig. 2). The primary endpoints were the intraoperative bleeding volume per 10 minutes and the degree of thecal sac compression by epidural hematoma. Statistical analysis was done using Student t-test for parametric variables and Fisher exact test for nonparametric variables for homogeneity comparison. Student t-test was used for comparison of the primary endpoints. The criterion for statistical significance (p-value) was set as < 0.05. SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA) was used for the analysis.
The two groups were homogenous regarding age and sex (demographic factors). The two groups were also homogenous with respect to the number of fusion segments, operation time, and primary/revision (surgical factors). PFA-EPI was delayed in the APDT group than in the NAPDT group (203.6 seconds vs. 170.0 seconds, p = 0.050). Other coagulation-related factors were homogenous between the groups (Table 3). Regarding the primary endpoints, bleeding volume per 10 minutes showed no difference between groups: 40.6 ± 12.8 mL in the APDT group and 43.9 ± 9.9 mL in the NAPDT group (p = 0.154). The cross-sectional area of the thecal sac at the maximal compression site also showed no difference: 120.2 ± 48.2 mm2 in the APDT group and 123.2 ± 50.4 mm2 in the NAPDT group (p = 0.766) (Table 4).
APDs have been used for primary prevention of cerebrovascular and cardiovascular diseases. It is essential to use APD to prevent restenosis after cardiovascular intervention. Dual antiplatelet therapy with aspirin and clopidogrel is the standard remedy for patients who experienced acute myocardial infarction or underwent coronary angioplasty and stent insertion.10) Among the patients who need noncardiac surgery, the prevalence of previous cerebrovascular and neurovascular diseases has been increasing. This is attributable to the growth of the advanced age population. Of the patients who underwent cardiac intervention, 26% undergo more than one noncardiac surgery within 5 years, and orthopedic surgery, which is required in 23%, is the most frequently performed surgery.11) In principle, elective surgery should be postponed for at least 1 year after coronary angioplasty with drug-eluting stents.12) Even after 1 year, it is recommended to maintain the aspirin therapy despite discontinuation of clopidogrel therapy.12) Therefore, it can be unavoidable to perform orthopedic surgery on patients taking APDs. However, in spine surgery, compression of nerve tissues by an epidural hematoma can cause a serious neurological complication; thus, most spine surgeons are reluctant to perform operations for APD users.13)
There are many reports that associate APD therapy with spontaneous or postinvasive procedural development of spinal epidural hematoma.89) However, there is no consensus on postoperative hematomas because there are equally many studies showing that POSEH and other bleeding-related complications did not increase in APD users.567) Postoperative epidural bleeding can be managed with use of suction drains unlike other cases of epidural bleeding. Rather than the simple increase of bleeding tendency, complicated interaction of factors may contribute to the development of POSEH. Increased bleeding tendency would not necessarily increase intraoperative bleeding. Because it is also influenced by the surgeon's bleeding control technique with help of an anesthesiologist. In this study, we attempted to compare the bleeding amount and POSEH between APDT and NAPDT groups in a homogenous cohort. We measured the total amount of bleeding and operation time and calculated the average bleeding amount per 10 minutes to avoid the confounding effect caused by the difference in the magnitude of surgery. It was difficult to measure three-dimensional volume of the total epidural hematoma and it was variable according to the number of operation segments. So, we thought it would be more meaningful to measure the cross-sectional area of the thecal sac where it was compressed maximally by POSEH. There was no difference in intraoperative hemorrhage per 10 minutes between the groups. As far as we know, there was no previous study that compared intraoperative bleeding per unit time. Soleman et al.7) also reported similar results by comparing intra- and postoperative bleeding between low-dose aspirin takers and non-takers in simple decompression surgery and there was no difference between the two groups in their study.
We presumed that postoperative epidural blood could be drained more efficiently through suction drains if platelet function decreases because blood clotting would be retarded. We did not measure the amount of drained blood. Park et al.14) measured drained blood amount through suction drains and supported our presumption: the total amount was greater in low dose aspirin users after posterior lumbar fusion surgery. In our study, postoperative thecal sac compression was not greater in the APDT group. Cuellar et al.5) also reported that aspirin did not increase symptomatic POSEH after spine surgery. It is not easy to determine statistical significance because the incidence of POSEH is generally very low. However, their study compared 100 cases in each group and there was no symptomatic POSEH in both groups. Leonardi et al.15) demonstrated in their prospective research that the size of POSEH and degree of thecal sac compression were positively correlated with neurological deficit. We thought that comparing the degree of thecal sac compression by measuring the cross-sectional area of the thecal sac at the maximal compression site by POSEH is more meaningful than comparing the neurological symptoms to evaluate the risk of POSEH because the incidence of symptomatic POSEH is too low. Fortunately, there was no patient who underwent a revision surgery due to a symptomatic POSEH in our cohort.
American College of Chest Physicians recommends discontinuance of aspirin 7 to 10 days before an elective surgery.16) There are many cases where APD was discontinued less than 7 days before surgery in the literature. Lee et al.17) conducted a prospective study to evaluate the recovery time of platelet function after discontinuation of aspirin and verified that platelet function began to recover after 72 hours and fully recovered at 96 hours. Based on their study, we classified those who discontinued APD less than 3 days before surgery as the APDT group and more than 4 days before surgery as the NAPDT group.
There are several limitations of this study. The sample size of the APDT group was relatively small. Patients eligible for inclusion in the NAPDT group were older and showed female predominance; thus, we excluded females less than 65 years from the NAPDT group to make a homogenous cohort. We could not compare the amount of drained blood because it was not our routine to record it. The strengths of this study include that bleeding per unit time was measured rather than the total amount of bleeding to eliminate the confounding effect of the magnitude of surgery and evaluated the degree of thecal sac compression as the primary end point to determine the effect of APD more precisely. Preoperative APD use did not increase intraoperative bleeding and POSEH. Therefore, it would be safer for patients who have cardiovascular and neurovascular risks to maintain APD therapy before spine surgery.
References
1. Chassot PG, Marcucci C, Delabays A, Spahn DR. Perioperative antiplatelet therapy. Am Fam Physician. 2010; 82(12):1484–1489. PMID: 21166368.
2. Komiya R, Kamintani K, Kubo E, et al. Perioperative management and postoperative complication rates of patients on dual antiplatelet therapies after coronary drug eluting stent implantation. Masui. 2014; 63(6):629–635. PMID: 24979851.
3. Wahl MJ. Dental surgery and antiplatelet agents: bleed or die. Am J Med. 2014; 127(4):260–267. PMID: 24333202.

4. Libanio D, Costa MN, Pimentel-Nunes P, Dinis-Ribeiro M. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc. 2016; 84(4):572–586. PMID: 27345132.
5. Cuellar JM, Petrizzo A, Vaswani R, Goldstein JA, Bendo JA. Does aspirin administration increase perioperative morbidity in patients with cardiac stents undergoing spinal surgery? Spine (Phila Pa 1976). 2015; 40(9):629–635. PMID: 26030214.

6. Smilowitz NR, Oberweis BS, Nukala S, et al. Perioperative antiplatelet therapy and cardiovascular outcomes in patients undergoing joint and spine surgery. J Clin Anesth. 2016; 35:163–169. PMID: 27871515.

7. Soleman J, Baumgarten P, Perrig WN, Fandino J, Fathi AR. Non-instrumented extradural lumbar spine surgery under low-dose acetylsalicylic acid: a comparative risk analysis study. Eur Spine J. 2016; 25(3):732–739. PMID: 25757534.

8. Mishima K, Aritake K, Morita A, Miyagawa N, Segawa H, Sano K. A case of acute spinal epidural hematoma in a patient with antiplatelet therapy. No Shinkei Geka. 1989; 17(9):849–853. PMID: 2797370.
9. Weber J, Hoch A, Kilisek L, Spring A. Spontaneous intraspinal epidural hematoma secondary to use of platelet aggregation inhibitors. Dtsch Med Wochenschr. 2001; 126(31-32):876–878. PMID: 11569370.
10. Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001; 345(7):494–502. PMID: 11519503.

11. To AC, Armstrong G, Zeng I, Webster MW. Noncardiac surgery and bleeding after percutaneous coronary intervention. Circ Cardiovasc Interv. 2009; 2(3):213–221. PMID: 20031718.

12. Chen TH, Matyal R. The management of antiplatelet therapy in patients with coronary stents undergoing noncardiac surgery. Semin Cardiothorac Vasc Anesth. 2010; 14(4):256–273. PMID: 21059610.

13. Korinth MC, Gilsbach JM, Weinzierl MR. Low-dose aspirin before spinal surgery: results of a survey among neurosurgeons in Germany. Eur Spine J. 2007; 16(3):365–372. PMID: 16953446.

14. Park JH, Ahn Y, Choi BS, et al. Antithrombotic effects of aspirin on 1- or 2-level lumbar spinal fusion surgery: a comparison between 2 groups discontinuing aspirin use before and after 7 days prior to surgery. Spine (Phila Pa 1976). 2013; 38(18):1561–1565. PMID: 23680836.
15. Leonardi MA, Zanetti M, Saupe N, Min K. Early postoperative MRI in detecting hematoma and dural compression after lumbar spinal decompression: prospective study of asymptomatic patients in comparison to patients requiring surgical revision. Eur Spine J. 2010; 19(12):2216–2222. PMID: 20556438.

16. Samama CM, Geerts WH. Prevention of intraoperative venous thromboembolism: what are the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition)? Ann Fr Anesth Reanim. 2009; 28(9 Suppl):S23–S28. PMID: 19875001.
17. Lee J, Kim JK, Kim JH, et al. Recovery time of platelet function after aspirin withdrawal. Curr Ther Res Clin Exp. 2014; 76:26–31. PMID: 25031665.

Fig. 1
Diagram of patient enrollment. There were total 449 cases that underwent a posterior thoracolumbar decompression and fusion surgery at our institution. Of those, 165 cases were excluded because they did not take postoperative magnetic resonance imaging. Among the remaining 284 cases, 34 cases were classified as antiplatelet drug takers (APDTs) and included in the study. Of the 250 non-APDTs (NAPDTs), 171 cases were excluded for the homogeneity of age and sex, and finally 79 NAPDTs were included. MRI: magnetic resonance imaging.
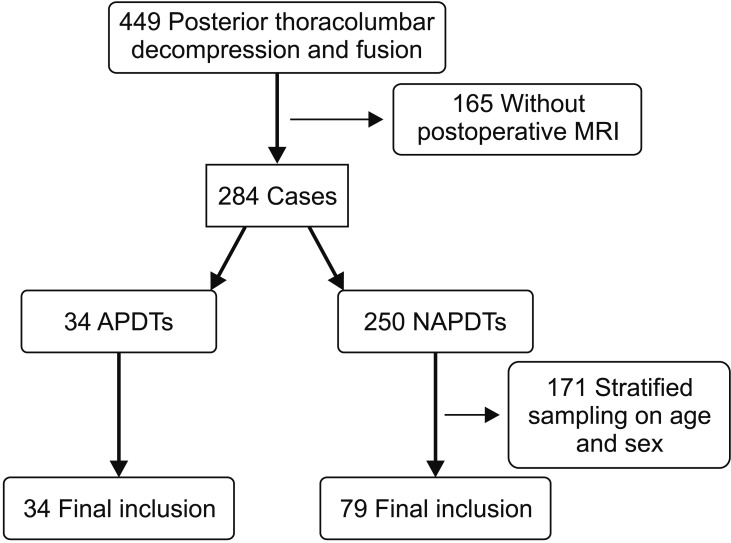
Fig. 2
Measurement of the cross-sectional area of the thecal sac. On T2-weighted axial image where the thecal sac was compressed maximally by epidural hematoma, the measurement was performed using free line region of interest (ROI) program in PACS (picture archiving and communication system).
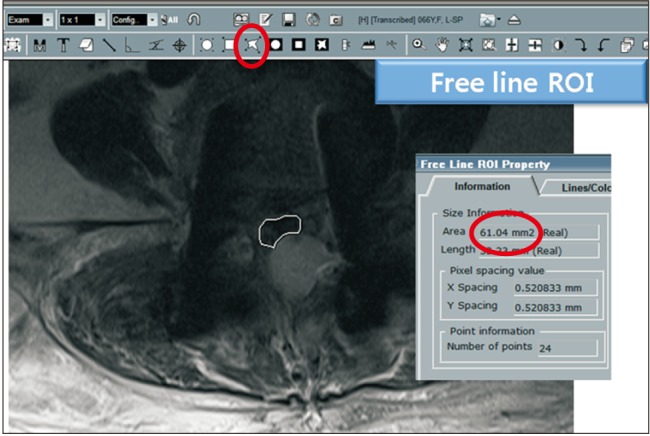
Table 1
Causes of Continuous Antiplatelet Drug Use
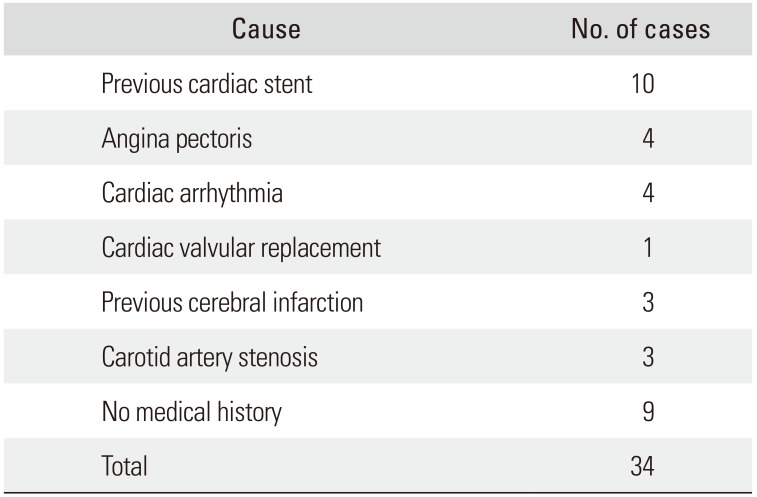
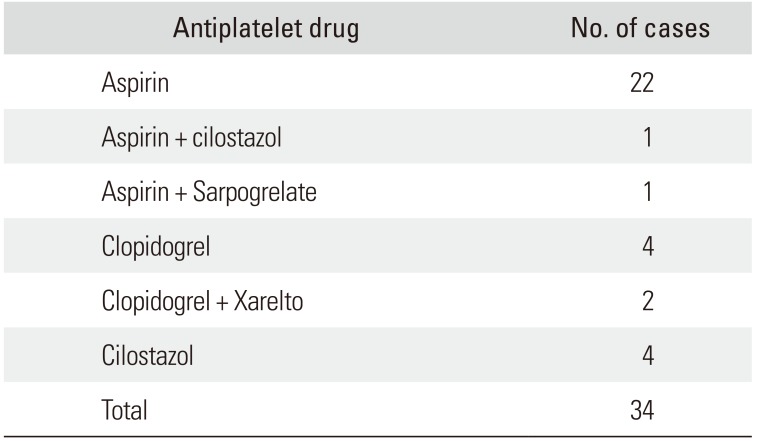
Table 2
Antiplatelet Drugs That Were Used
| Antiplatelet drug | No. of cases |
|---|---|
| Aspirin | 22 |
| Aspirin + cilostazol | 1 |
| Aspirin + Sarpogrelate | 1 |
| Clopidogrel | 4 |
| Clopidogrel + Xarelto | 2 |
| Cilostazol | 4 |
| Total | 34 |
Table 3
Demographic Data




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download