Abstract
Background
Open microscopic laminectomy has been the standard surgical method for degenerative spinal stenosis without instability till now. However, it is associated with complications such as paraspinal muscle injury, excessive bleeding, and wound infection. Several surgical techniques, including microendoscopic decompression, have been introduced to solve these problems.
Methods
Authors analyzed retrospectively 55 patients presenting with neurological symptoms due to degenerative lumbar spinal stenosis refractory to conservative treatment. Patients with foraminal stenosis requiring foraminal decompression were excluded. Two or three portals were used for each level. One portal was used for viewing purpose and the others for instrument passage. Unilateral laminotomy was followed by bilateral decompression under the view of 30° arthroscopy. Clinical outcomes were evaluated using modified Macnab criteria, Oswestry disability index (ODI), and visual analogue scale (VAS). Postoperative complications were checked during the 2-year follow-up. Plain radiographs before and after surgery were compared to analyze the change of disc height decrement and alignment.
Results
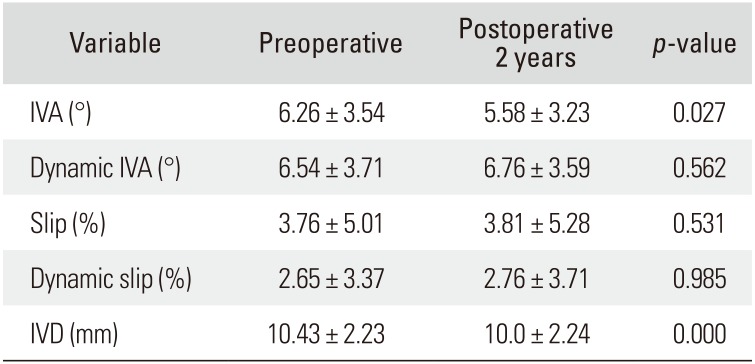
ODI scores improved from 67.4 ± 11.5 preoperatively to 19.3 ± 12.1 at 2-year follow-up (p < 0.01). VAS scores of the leg decreased from 7.7 ± 1.5 to 1.7 ± 1.5 at the final follow-up (p < 0.01). Per the modified Macnab criteria, 81% of the patients improved to good/excellent. No cases of infection occurred. The intervertebral angle was significantly reduced from 6.26° ± 3.54° to 5.58° ± 3.23° at 2 years postoperatively (p = 0.027) and the dynamic intervertebral angle changed from 6.54° ± 3.71° to 6.76° ± 3.59°, which was not statistically significant (p = 0.562). No significant change in slippage was observed (3.76% ± 5.01% preoperatively vs. 3.81% ± 5.28% at the final follow-up [p = 0.531]). The dynamic percentage slip did not change significantly, from 2.65% ± 3.37% to 2.76% ± 3.71% (p = 0.985). However, intervertebral distance decreased significantly from 10.43 ± 2.23 mm to 10.0 ± 2.24 mm (p = 0.000).
Conclusions
Full endoscopic decompression using a 30° arthroscopy demonstrated a satisfactory clinical outcome at the 2-year follow-up. This technique reduces wound infection rate and did not bring about postoperative segmental spinal instability. It could be a feasible alternative to conventional open microscopic decompression or fusion surgery for degenerative lumbar spinal stenosis.
Spinal stenosis occurs when the neural structure is encroached on by surrounding soft tissue and bone.123) Lumbar spinal stenosis is more prevalent in the elderly. Open laminectomy and spinal fusion had been standard surgical treatment methods for various types of lumbar spinal stenosis.4) However, these procedures can cause several complications. The standard surgery procedure involves wide dissection of the paraspinal muscles to create an operating space, followed by removal of excessive bone and ligament for decompression.5) Firm fusion can accelerate degeneration of the adjacent unfused segment.4) Adjacent segmental degeneration can also occur due to wide posterior dissection that injures the paraspinal muscles during posterior lumbar fusion. Multifidus muscle injury and atrophy are common after posterior lumbar spine surgery and are associated with lower back pain and functional disability. Thus, microscopic bilateral decompression has been suggested as an alternative to open laminectomy and fusion. Several studies have reported favorable long-term results,67891011) and the technique is currently considered the standard technique. Recently, bilateral decompression using unilateral biportal endoscopy has been reported by several authors and has begun to attract attention.1213) So far, there are few studies on patients with a follow-up of more than 2 years after unilateral biportal endoscopic spinal surgery. This study evaluates the short-term outcome of unilateral biportal endoscopic spinal surgery for degenerative spinal stenosis performed with 30° arthroscopy.
We conducted this study in compliance with the principles of the Declaration of Helsinki. The protocol of this study was reviewed and approved by the Institutional Review Board of Andong Hospital (IRB No. 2018-003). We received informed consent from all patients before surgery. All patients who underwent unilateral biportal endoscopic decompression (UBED) provided a signed consent form prior to surgery. The clinical outcomes including Oswestry Disability Index (ODI), Modified Macnab criteria, visual analogue scale (VAS), operation time, and complication rate were analyzed in patients who were treated with UBED using 30° arthroscopy. Authors compared plain radiographs taken before and 2 years after surgery. Plain radiographs of flexion and extension postures before and after surgery were compared and analyzed to confirm disc height reduction, percentage of slip, and intervertebral angles. Intervertebral distance was measured between two vertebrae at the midpoint of the end plates (Fig. 1). Among the 91 patients that underwent UBED from our institute, patients that were lost during follow-up and patients with other diagnoses such as lumbar disc herniation, adjacent segmental degeneration, and moderate or higher grade foraminal stenosis were excluded; eventually 55 patients were enrolled in this study (Fig. 2). The index level was L2–3 in three cases (5.4%), L3–4 in 13 (23.6%), L4–5 in 29 (52.7%), and L5–S1 in 10 (18.1%). The main symptom of the patients was neurologic claudication in 43 cases (78.1%) and paresthesia in 12 cases (21.8%). No cases had lower extremity weakness or back pain as a main symptom (Table 1). UBED was performed by a single surgeon (JEK). Basic spine surgery instruments, including a 30° 4-mm arthroscopy, which is commonly used in joint arthroscopic surgery, and a radiofrequency, 4.2-mm arthroscopic burr, along with a shaver, were used for surgery. Surgery was performed under spinal or general anesthesia with the patient in the prone abdomen free position so that the spine could be flexed to widen the interlaminar spaces. Spinal anesthesia was performed only for the index level of L4–5 or L5–S1 when the patient requested.
Level confirmation was conducted using the C-arm before surgery. On the C-arm anteroposterior view, a proximal portal was created just below the pedicle level of the lamina that was to be laminotomized; a distal portal was created just below the pedicle level of the lamina 1 level below. The two portals were 0.5 cm in length, large enough to insert instruments and endoscope. The distance between two portals differed depending on patient height and level. The distal portal was located at an average of 2 cm caudal to the proximal portal (Fig. 3).
The trocar of the scope was introduced into the proximal portal and a round, smooth periosteal elevator was inserted into the distal portal through the paraspinal muscle without any dissection until it was situated at the end of the lamina. The muscle overlying the lamina and interlaminar space was then pushed aside using the periosteal elevator to create a visual surgical field. Water inflow with a pressure of less than 30 mmHg was initiated through the proximal portal. The pressure of water inflow could be achieved using gravity instead of an infusion pump. The outflow of the water was maintained throughout the procedure directing through the distal portal to achieve continuous irrigation and prevent soft tissue congestion due to water accumulation. Identification of the facet joint, which is a significant landmark during surgery, was performed. The lamina was identified along the facet joint. A shaver or radiofrequency catheter was used to clean the soft tissue and debris over the lamina. The arthroscopic 4.2-mm burr or chisel was used to thin out the ipsilateral lamina and a part of the spinous process, which was followed by remnant laminectomy by the Kerrison punch to achieve a laminotomy. Partial laminectomy was performed to expose the ligament flavum sufficiently before flavectomy. The partial medial facetectomy was performed if the lateral recess was compressed according to magnetic resonance imaging (MRI). The ligamentum flavum was not resected as it functions as a protective barrier until the bony work at the contralateral lamina was completed. During 30° arthroscopy, the 12 o'clock position was used to visualize the base of the contralateral lamina and the spinous process, while the 6 o'clock angle was used to visualize the inferior articular process and inferior lamina. Ipsilateral decompression was performed with an arthroscopic burr and Kerrison rongeur until the proximal end of the ligamentum flavum was exposed, followed by undercutting of the contralateral lamina and the base of the spinous process to create sufficient space to handle the contralateral lamina. After confirming there was no adhesion between the ligamentum flavum and dura, flavectomy was performed using the Kerrison punch and pituitary forceps for ipsilateral neural structure decompression. For contralateral side decompression, both the arthroscopy and working instrument should pass the midline to the contralateral lamina to approach the contralateral ligamentum flavum. A smooth curette was used to detach the ligamentum flavum from the contralateral lamina wall and to create a free space between the dura and ligamentum flavum to prevent iatrogenic durotomy during flavectomy. The ligamentum flavum was removed using a Kerrison punch and pituitary forceps only after complete detachment from both the dura and lamina wall. If the bony work or flavectomy was not done in the desired direction during the operation, an auxiliary portal was made below 1.5 cm distal from the working portal. Surgery was considered complete after confirmation of bilateral decompression using a blunt probe. After removing the instruments and arthroscope, the skin was repaired (Fig. 4). Postoperative MRI was performed after drain removal.
A total of 55 patients (26 males and 29 females; mean age, 70.7 ± 14.3 years; range, 55 to 86 years) were enrolled in this study. Blood loss could not be accurately checked due to the continuous water irrigation. The mean follow-up period was 29 months. The mean operative time for 1 level was 53 ± 11.5 minutes. Complications occurred in three cases: two cases of dural tear and one case of epidural hematoma. Regarding the two dural tear cases, authors immediately recognized them intraoperatively, and they were both small sized tears (less than 5 mm). The patients complained of headache the day after the operation, which improved after 3 days of bed rest. One patient who developed neurological symptoms due to postoperative epidural hematoma underwent revision surgery. Endoscopic hematoma evacuation was performed using the portal used in previous surgery instead of an open surgery. All postoperative complications occurred within 1 week after surgery; no revision surgery was necessary afterwards (Table 2). The mean VAS of the legs improved from 7.7 ± 1.5 preoperatively to 3.1 ± 1.3 after surgery (p < 0.01); it was 1.7 ± 1.5 (p < 0.01) at the 2-year follow-up. The postoperative mean ODI significantly improved from 67.4 ± 11.5 to 28.5 ± 13.2 (p < 0.01); it was 19.3 ± 12.1 (p < 0.01) at the 2-year follow-up. Among the patients, 81% reported improvement per the Macnab criteria; the recorded outcomes were excellent in 25 patient (45%), good in 20 (36%), fair in nine (16%), and poor in one (2%) (Fig. 5).
The intervertebral angle reduced significantly from 6.26° ± 3.54° to 5.58° ± 3.23° at 2 years postoperatively (p = 0.027). The dynamic intervertebral angle decreased slightly to 6.54° ± 3.71° from 6.76° ± 3.59°, but the difference was not statistically significant (p = 0.562). Preoperative percentage of slip was 3.76% ± 5.01%, with a slip of 3.81% ± 5.28% at the 2-year follow-up (p = 0.531), which was not a significant change. The preoperative dynamic percentage of slip (gap in the percentage of slip between the flexion and extension views) was 2.65% ± 3.37% preoperatively and at 2 years postoperatively, it was 2.76% ± 3.71% (p = 0.985), which was not a significant change. However, at 2 years postoperatively, the intervertebral distance reduced significantly from 10.43 ± 2.23 mm to 10.0 ± 2.24 mm (p = 0.000) (Table 3). Pre- and postoperative MRI and radiological changes in one of the enrolled patients are shown in Fig. 6.
The surgical goal of treatment for symptomatic lumbar spinal stenosis is alleviation of symptoms through proper neural decompression while preserving the original anatomy and biomechanics of the spine. Several surgical techniques have hitherto been used for the same purpose.131415) Although traditional open laminectomy can be used to treat lumbar spinal stenosis with excellent results in 64% of the cases,16) back pain and muscle atrophy can be caused by excessive dissection of the paraspinal muscle.2) Bony destruction due to open laminectomy can cause spinal column instability.17) The number of posterior lumbar fusions also increases due to the inevitable iatrogenic instability that is caused by wide decompression including facetectomy.18) Several studies have reported that a large dead space due to open spinal surgery increases the infection rate or contributes to scarring on neural structures.6819) Furthermore, adjacent segmental degeneration can occur after posterior lumbar fusion. These complications can lead to chronic back pain or failed back syndrome.20)
Unilateral laminectomy bilateral decompression (ULBD) has been used to achieve bilateral decompression without contralateral dissection as well as to reduce muscle dissection with satisfactory results.78911) The concept of minimally invasive surgery has been applied to various surgical fields and has shown good results. The clinical outcome of ULBD using microscopy has been satisfactory in several studies.11202122) The decompression method using the unilateral biportal endoscopic technique was then introduced.1213) However, till now, the clinical and radiological reports after more than 2 years of follow-up after UBED for degenerative lumbar spinal stenosis have not been studied.
The results of our series were relatively superior to data shown in other studies on conventional open laminectomy with wider dissection and laminectomy, which have a success rate of 56%–64%.16) A clinical study on bilateral laminotomy showed a success rate of about 90%23) and another showed a success rate of 80%.24) A study on ULBD demonstrated a success rate of approximately 67.6%–81%.6252627) Our study showed results similar to those obtained in a study of Pao et al.11) on patients who underwent microendoscopic decompressive laminectomy, a similar minimally invasive technique. In the study, more than 80% of the patients reported good or excellent results.
In our study, a significant improvement was seen in the ODI score in patients undergoing UBED, and > 81% of patients reported good or excellent scores per the modified Macnab criteria, which is an indicator of patient satisfaction. There was no significant difference in the results of unilateral and bilateral laminotomy. The UBED is as effective as the microendoscopic decompressive laminectomy in relieving neurological symptoms and improving patient life quality. There are several factors that are responsible for this. First, since 30° arthroscopy was used, it was possible to view the spine from various angles, enabling complete decompression of the neural structures. Second, the muscle damage was minimal. Unlike open surgery, there were few potential muscle injuries because the procedure was performed percutaneously with a small incision. Decompression was sufficiently performed under an enlarged arthroscopic field, reducing iatrogenic laminar fractures and excessive facet injuries.
Although the concept of preservation of the spinal structure is considered important in decompression surgery using the minimally invasive technique, there has always been concern about incomplete decompression in this technique. However, the symptomatic improvement was satisfactory immediately after the operation, and the results were satisfactory even after 2 years in this study. The fact that the symptoms did not deteriorate after 2 years shows that there was sufficient decompression through good visualization in arthroscopy, and restenosis did not occur even 2 years after surgery.
Postoperative instability due to laminectomy is a major concern in decompression surgery. In our study, 17 of 55 patients were diagnosed with grade I mild degenerative spondylolisthesis, but no postoperative instability occurred at the last follow-up. This is mostly because dynamic intervertebral angle and dynamic slip percentage did not significantly increase after the operation. Another reason for the absence of lumbar instability is that this technique is less invasive, minimizing destruction of posterior elements and facet joints. UBED could limit facet destruction and prevent excessive resection of the bone in a magnified operation field. In Oertel et al.'s study,28) instability occurred in only two of 133 patients with spinal stenosis who underwent ULBD. Sasai et al.'s study10) demonstrated that the increases in dynamic intervertebral angles and dynamic slip were not statistically significant in patients undergoing a microscopic bilateral decompression via the unilateral approach. Our study confirmed that UBED is a good surgical option to decompress the stenosis while preserving the intrinsic stabilizer.
The UBED technique allows decompression of the contralateral side and identification of the contralateral facet as well as the traversing root without excessive resection of the bony structure. The portals created in the intermuscular plane of the multifidus provide space between the multifidus and the lamina in order to prevent damage to the posterior ligament complex and the paraspinal muscle. Although the prevalence of spinal surgical site infection was reported to be 3.5% in a validation cohort study,29) our study did not have a single case of infection. There were no complications associated with general anesthesia such as pneumonia due to the relatively short operation time. Intraoperative bony and soft tissue debris did not create any congestion due to the continuous water irrigation, which could also be a factor reducing the possibility of infection. Authors assumed that the inflammatory environment did not develop due to continuous saline irrigation.
Unintentional dural tears are another concern during decompression surgery although long-term results are mostly positive.30) The frequency of dural tears was lower in UBED (3.6%) than in microendoscopic lumbar decompression (8.3%)11) and in open laminectomy (18%).16) Regarding UBED, the visual field is relatively good because arthroscopy is used. The possibility of dural tears is also likely to be reduced because the contralateral side can be easily accessed. In our study, dural tears occurred in two patients early in the learning curve, and fortunately the tears were within 5 mm. Both patients showed symptomatic improvement after conservative treatment 3 days after operation. No revision surgeries have been needed due to dura tears so far. No patient required transfusion due to postoperative bleeding.
There are some limitations to this study. The first is the absence of a control group for comparison. Second, the follow-up is not long enough to conclusively determine the long-term benefits. Third, this study did not include patients diagnosed with higher grade spondylolisthesis. UBED using 30° arthroscopy provided favorable clinical outcomes, and postoperative instability did not occur after 2 years. It can be a useful procedure to reduce surgical infection through continuous irrigation and prevent postoperative segmental spinal instability. Therefore, UBED could be an alternative minimally invasive spine surgery method instead of conventional open decompression or fusion surgery for treating degenerative lumbar spinal stenosis.
References
1. Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008; 358(8):794–810. PMID: 18287602.

2. Hu ZJ, Fang XQ, Zhou ZJ, Wang JY, Zhao FD, Fan SW. Effect and possible mechanism of muscle-splitting approach on multifidus muscle injury and atrophy after posterior lumbar spine surgery. J Bone Joint Surg Am. 2013; 95(24):e192(1-9). PMID: 24352778.

3. Huang YH, Ou CY. Significant blood loss in lumbar fusion surgery for degenerative spine. World Neurosurg. 2015; 84(3):780–785. PMID: 25986203.

4. Kim JY, Ryu DS, Paik HK, et al. Paraspinal muscle, facet joint, and disc problems: risk factors for adjacent segment degeneration after lumbar fusion. Spine J. 2016; 16(7):867–875. PMID: 26970600.

5. Iguchi T, Kurihara A, Nakayama J, Sato K, Kurosaka M, Yamasaki K. Minimum 10-year outcome of decompressive laminectomy for degenerative lumbar spinal stenosis. Spine (Phila Pa 1976). 2000; 25(14):1754–1759. PMID: 10888941.

6. Mariconda M, Fava R, Gatto A, Longo C, Milano C. Unilateral laminectomy for bilateral decompression of lumbar spinal stenosis: a prospective comparative study with conservatively treated patients. J Spinal Disord Tech. 2002; 15(1):39–46. PMID: 11891449.

7. Palmer S, Turner R, Palmer R. Bilateral decompression of lumbar spinal stenosis involving a unilateral approach with microscope and tubular retractor system. J Neurosurg. 2002; 97(2 Suppl):213–217. PMID: 12296681.

8. Cavusoglu H, Kaya RA, Turkmenoglu ON, Tuncer C, Colak I, Aydin Y. Midterm outcome after unilateral approach for bilateral decompression of lumbar spinal stenosis: 5-year prospective study. Eur Spine J. 2007; 16(12):2133–2142. PMID: 17712577.

9. Costa F, Sassi M, Cardia A, et al. Degenerative lumbar spinal stenosis: analysis of results in a series of 374 patients treated with unilateral laminotomy for bilateral microdecompression. J Neurosurg Spine. 2007; 7(6):579–586. PMID: 18074681.

10. Sasai K, Umeda M, Maruyama T, Wakabayashi E, Iida H. Microsurgical bilateral decompression via a unilateral approach for lumbar spinal canal stenosis including degenerative spondylolisthesis. J Neurosurg Spine. 2008; 9(6):554–559. PMID: 19035747.

11. Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J. 2009; 18(5):672–678. PMID: 19238459.

12. Choi CM, Chung JT, Lee SJ, Choi DJ. How I do it? Biportal endoscopic spinal surgery (BESS) for treatment of lumbar spinal stenosis. Acta Neurochir (Wien). 2016; 158(3):459–463. PMID: 26782827.

13. Hwa Eum J, Hwa Heo D, Son SK, Park CK. Percutaneous biportal endoscopic decompression for lumbar spinal stenosis: a technical note and preliminary clinical results. J Neurosurg Spine. 2016; 24(4):602–607. PMID: 26722954.

14. Baghdadi YM, Moussallem CD, Shuaib MA, Clarke MJ, Dekutoski MB, Nassr AN. Lumbar spinous process-splitting laminoplasty: a novel technique for minimally invasive lumbar decompression. Orthopedics. 2016; 39(5):e950–e956. PMID: 27337665.

15. Young S, Veerapen R, O'Laoire SA. Relief of lumbar canal stenosis using multilevel subarticular fenestrations as an alternative to wide laminectomy: preliminary report. Neurosurgery. 1988; 23(5):628–633. PMID: 3200393.

16. Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis: attempted meta-analysis of the literature. Spine (Phila Pa 1976). 1992; 17(1):1–8. PMID: 1531550.
17. Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976). 2004; 29(7):726–733. PMID: 15087793.
18. Lipson SJ. Spinal-fusion surgery: advances and concerns. N Engl J Med. 2004; 350(7):643–644. PMID: 14960739.
19. Jayarao M, Chin LS. Results after lumbar decompression with and without discectomy: comparison of the transspinous and conventional approaches. Neurosurgery. 2010; 66(3 Suppl Operative):152–160. PMID: 20173565.

20. Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014; 21(2):179–186. PMID: 24878273.
21. Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine (Phila Pa 1976). 2002; 27(4):432–438. PMID: 11840112.

22. Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002; 51(5 Suppl):S146–S154. PMID: 12234442.

23. Lin PM. Internal decompression for multiple levels of lumbar spinal stenosis: a technical note. Neurosurgery. 1982; 11(4):546–549. PMID: 7145072.

24. Aryanpur J, Ducker T. Multilevel lumbar laminotomies: an alternative to laminectomy in the treatment of lumbar stenosis. Neurosurgery. 1990; 26(3):429–432. PMID: 2138716.

25. Weiner BK, Walker M, Brower RS, McCulloch JA. Microdecompression for lumbar spinal canal stenosis. Spine (Phila Pa 1976). 1999; 24(21):2268–2272. PMID: 10562995.

26. Spetzger U, Bertalanffy H, Reinges MH, Gilsbach JM. Unilateral laminotomy for bilateral decompression of lumbar spinal stenosis. Part II: clinical experiences. Acta Neurochir (Wien). 1997; 139(5):397–403. PMID: 9204107.

27. Thome C, Zevgaridis D, Leheta O, et al. Outcome after less-invasive decompression of lumbar spinal stenosis: a randomized comparison of unilateral laminotomy, bilateral laminotomy, and laminectomy. J Neurosurg Spine. 2005; 3(2):129–141. PMID: 16370302.
28. Oertel MF, Ryang YM, Korinth MC, Gilsbach JM, Rohde V. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery. 2006; 59(6):1264–1269. PMID: 17277689.

29. Klemencsics I, Lazary A, Szoverfi Z, Bozsodi A, Eltes P, Varga PP. Risk factors for surgical site infection in elective routine degenerative lumbar surgeries. Spine J. 2016; 16(11):1377–1383. PMID: 27520077.

30. Cammisa FP Jr, Girardi FP, Sangani PK, Parvataneni HK, Cadag S, Sandhu HS. Incidental durotomy in spine surgery. Spine (Phila Pa 1976). 2000; 25(20):2663–2667. PMID: 11034653.

Fig. 1
Measurement of radiological data. (A) Intervertebral distance: vertical distance between the vertebrae at 50% (white line) of the anteroposterior diameter of each vertebral body (gray line). (B) Slip percentage: slip% = (B− A / B) × 100. White line: anteroposterior diameter of the vertebral body of the lower vertebra. Gray line: anteroposterior diameter of the vertebral body of the upper vertebra. Dotted line: end of vertebra. (C) Intervertebral angle.
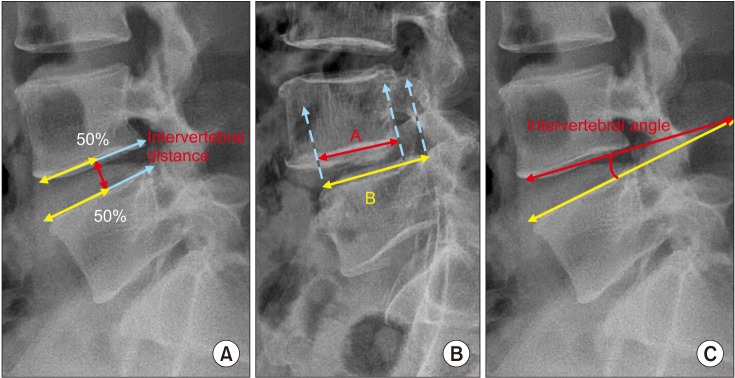
Fig. 3
(A) Setup for unilateral biportal endoscopic decompression surgery. (B) Locations of viewing (upper) and working (lower) portals. (C) Intraoperative photo showing the arthroscope and arthroscopic burr introduced through viewing and working portals.
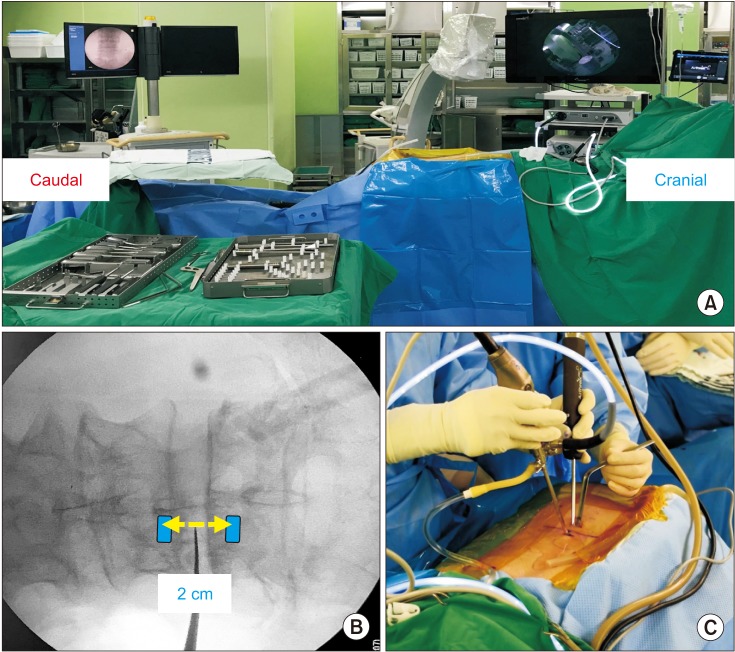
Fig. 4
(A) Intraoperative endoscopic image showing decompression of the central canal and ipsilateral laminotomy. (B) Postoperative photo showing the skin incision point in unilateral biportal endoscopic decompression.
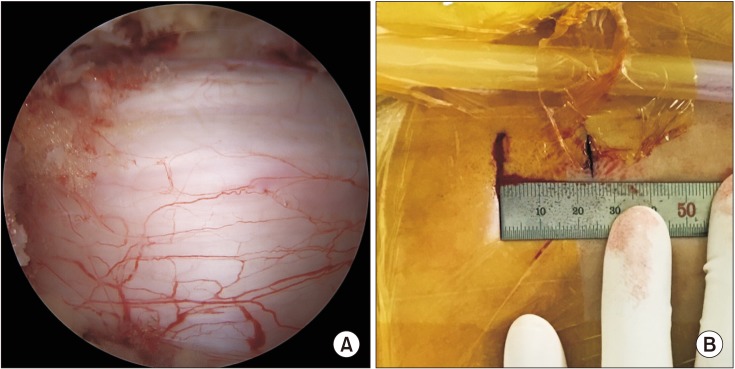
Fig. 5
(A) Visual analogue scale (VAS) pain scores assessed preoperatively and at 2 months and 2 years postoperatively. (B) Oswestry disability index (ODI) scores assessed preoperatively and at 2 months and 2 years postoperatively. (C) According to modified Macnab criteria, 81% of patients who underwent unilateral biportal endoscopic decompression experienced excellent or good results.
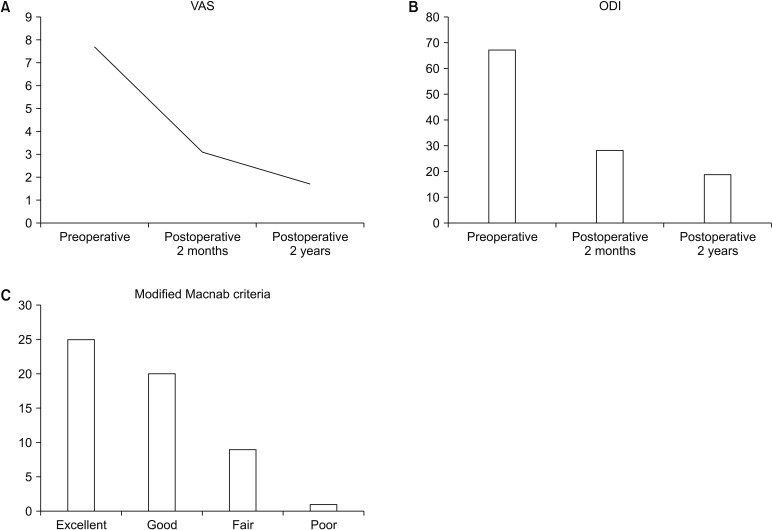
Fig. 6
Preoperative (A, B) and postoperative (C, D) T2-weighted magnetic resonance imaging scans obtained in a patient who underwent L4–5 decompression.
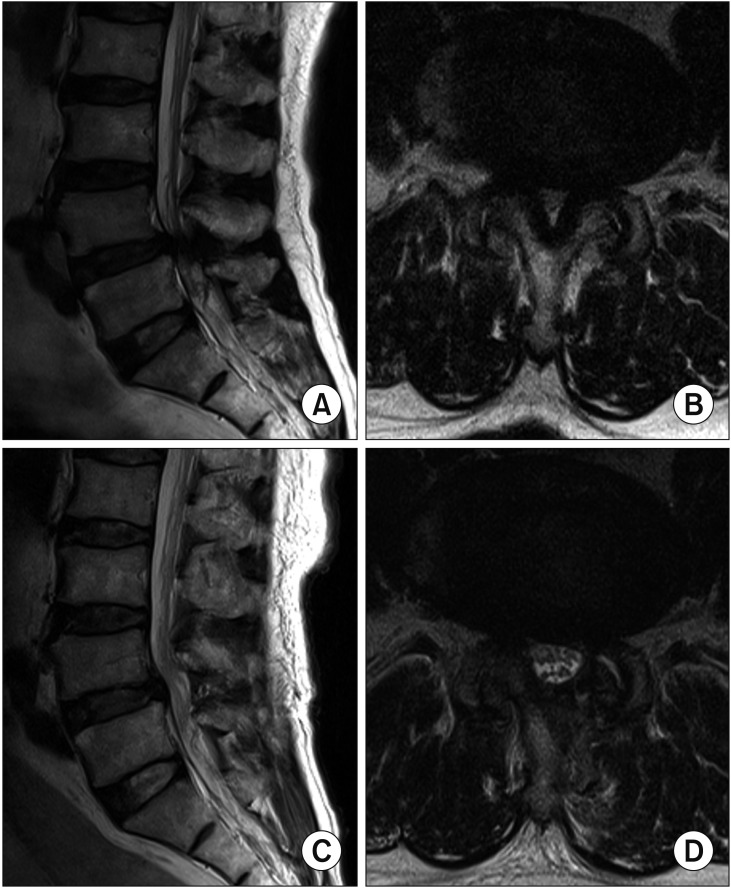
Table 1
Treated Disc Level and Main Patient Symptom

Table 2
Clinical and Demographic Data of Patients
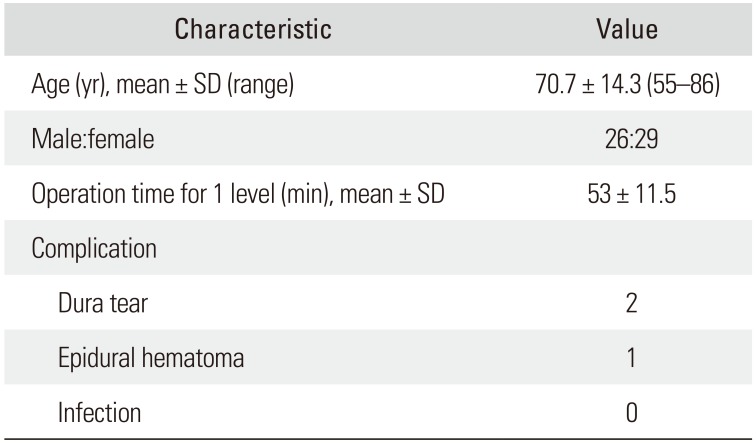
| Characteristic | Value |
|---|---|
| Age (yr), mean ± SD (range) | 70.7 ± 14.3 (55–86) |
| Male:female | 26:29 |
| Operation time for 1 level (min), mean ± SD | 53 ± 11.5 |
| Complication | |
| Dura tear | 2 |
| Epidural hematoma | 1 |
| Infection | 0 |
Table 3
Changes in Radiological Parameters of Preoperative and Postoperative 2 Years




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download