Abstract
Objective
Mirror aneurysms are generally considered as a subset of multiple aneurysms, defined as aneurysms occurring bilaterally and symmetrically on the same-named vessels. Although not infrequent, the characteristics of mirror aneurysms are not well studied. This investigation was conducted to elucidate the anatomic features of such lesions and examine treatment options.
Materials and Methods
A retrospective review was conducted, aimed at 172 patients treated for 344 mirror aneurysms between January 2007 and December 2015. Aneurysms of similar nature but in asymmetric locations on the same-named vessels were excluded. All available records were examined and lesion characteristics, as well as treatment outcomes were assessed.
Results
In study subjects (n = 172), mirror aneurysms most often involved middle cerebral artery bifurcation (n = 83), followed by a paraclinoid internal carotid artery (n = 50) and posterior communicating artery (n = 21). Most of the lesions (95.3%) measured ≤ 10 mm, and in 126 patients (74.6%), the size ratios were > 50%. Of the 344 aneurysms studied, coil embolization was undertaken in 217, surgical clipping in 62, and observation alone (no treatment) in 65. Coil embolization and surgical clipping were done bilaterally in 83 and 12 patients, respectively. In 12 patients, combined coiling and clipping were implemented on each side. Single-stage coil embolization of both the aneurysms was performed in 73 patients, with excellent post-procedural (85.6%) and follow-up (86.8%) occlusive results. There was no procedure-related morbidity or mortality.
The incidence of intracranial aneurysms in the general population is approximately 2–3.2% (1234), with multiple aneurysms accounting for 15–30% of all intracranial aneurysms (5678910). As cited in previous reports, risk factors for multiple aneurysms include female sex, history of smoking, hypertension, postmenopausal hormonal replacement therapy, and family history of subarachnoid hemorrhage (SAH) (5678910). Mirror aneurysms are a subset of multiple aneurysms that comprise 2–12% of all intracranial aneurysms. Different terminologies for mirror aneurysms have been described in previous reports (i.e., mirror intracranial multiple aneurysms, mirror-like intracranial aneurysm, and intracranial mirror aneurysm) along with different definitions (111213141516). In the present study, the term ‘a mirror aneurysm’ was employed and defined as ‘aneurysms developing bilaterally and symmetrically on same-named vessels.’
Two large analyses in the past have focused on sites of involvement and clinical characteristics of a mirror aneurysm. Meissner et al. (11) reported that patients with a mirror aneurysm were more likely to be women with a family history of aneurysmal SAH and with a tendency to develop larger lesions (compared with non-mirror counterparts). In addition, they reported middle cerebral artery (MCA) as the most common site of mirror aneurysms, followed by a non-cavernous internal carotid artery (ICA), posterior communicating artery (PcomA), and cavernous ICA. However, the findings of Lee et al. (12) were controversial. As per the study, there existed a difference of opinion regarding female predisposition and a family history of intracranial aneurysm and cited to be similar to non-mirror aneurysms; and a predilection shown for cavernous ICA was contrary to the results of Meissner et al. (11).
Despite abundance in the documentation on mirror aneurysms in terms of incidence, locations, and risk factors, only a few case reports and small-sized studies have addressed the management of mirror aneurysms (17181920). The purpose of this study was to investigate safety and feasibility of options for managing mirror aneurysms with emphasis on bilateral single-stage coil embolization. Furthermore, the angio-anatomic characteristics of the lesions were explored.
Between January 2007 and December 2015, 3432 consecutive patients with intracranial aneurysms underwent surgical clipping (n = 624) or endovascular management (n = 2808) at our neurovascular center. From a total of 3432 patients, 998 patients (29.1%) had multiple intracranial aneurysms and 172 patients (5.0% of patients overall and 17.2% of patients with multiple aneurysms) had mirror aneurysms (Fig. 1). Mirror aneurysms were defined as aneurysms developing bilaterally and symmetrically on same-named vessels. All aneurysms in patients selected for this study measured ≥ 2 mm in maximum diameter. However, similar lesions at asymmetric locations (on same-named vessels) were excluded. Therapeutic alternatives in mirror aneurysms were deliberated by neurosurgical/neurointerventional teams in a multidisciplinary decision-making process with the following strategy; 1) in unruptured cases, a single-stage coil embolization was preferred if technically possible, 2) if not, to avoid bilateral craniotomy, at least one of two aneurysms were treated with coil embolization, and 3) in patients who presented with a hemorrhage, staged treatment was preferred. At a stage, when there is a dilemma regarding the lesion rupture, both aneurysms should be simultaneously managed. This study complied with principles outlined in the Declaration of Helsinki and was approved by our Institutional Review Board.
Medical charts and imaging studies were reviewed by considering age of patients; sex; status at presentation (SAH or unruptured intracranial aneurysm); sites involved; treatment modalities; multiplicity of aneurysms; size of aneurysms; depth-to-neck ratio (D/N ratio); and outcomes (clinical and radiological), both immediate and extended. All aneurysms were detected using computed tomography angiography (CTA) or magnetic resonance angiography (MRA). In each instance, cerebral angiography and rotational angiography with 3-dimensional (3D) image reconstruction were undertaken via Integris V, Allura Clarity (both Philips Medical Systems, Best, The Netherlands), or Innova IGS 630 (GE Healthcare, Wauwatosa, WI, USA) units to assess aneurysmal configurations with precision and determine therapeutic plans. The size of the aneurysm (maximum diameters) was gauged through 3D angiograms. Subsequently, we compared mirror aneurysms at each location by computing a ratio of smaller to larger lesions. D/N ratios were measured in working projections of conventional angiography. In three instances, clipped aneurysms could not be measured (no baseline data). The patients had histories of unilateral clipping done elsewhere. Procedural time was recorded as the duration from a femoral puncture to arterial closure.
Immediate and delayed procedural complications were also investigated similarly to clinical outcomes. The complications were independently assessed by both neurovascular surgeons and neurointerventionist at the time of discharge and at follow-up visits to our outpatient clinic, using the modified Rankin Scale (mRS) to gauge patient's status. In the event of disagreement, we recorded the mRS assessed by a neurosurgeon who was not involved in the procedure.
In coil embolization procedures, initial angiographic outcomes were designated (according to Raymond classification) as complete occlusion, residual neck, or residual sac (21). The degree of recanalization was also determined at 6, 12, 24, and 36 months after endovascular treatment via MRA (22). Conventional angiography was recommended, if MRA was infeasible or if aneurysmal recanalization was suspected by MRA, rendering decisions on further treatment as needed. Recanalization status was stratified in follow-up images based on a 3-point Raymond scale as follows: 1) complete occlusion, 2) minor recanalization, or 3) major recanalization. Repeat embolization was advised in instances of major recanalization (21).
In surgical clipping procedures, estimates of initial aneurysmal outcomes (complete occlusion, remnant neck, or remnant sac) were obtained before discharge by CTA or by conventional angiography. Radiologic follow-up was not routinely recommended.
Overall, 172 patients with 344 mirror aneurysms were evaluated. Among them, 170 patients had mirror aneurysms at the time of initial diagnosis and two patients had single aneurysms initially, with de novo contralateral aneurysms developing during post-treatment monitoring. Seventy-two patients had additional unpaired aneurysms, involving a total of three aneurysms in 55 patients and ≥ 4 aneurysms in 17 patients (Fig. 1). There were 144 females (83.7%) and 28 males (16.3%) and the mean age was 58.7 ± 10.4 years (range, 24–85 years). Some patients (n = 18) presented with SAH, but in 154 patients, the mirror aneurysms were unruptured. The most frequent site of paired aneurysms was MCA bifurcation (n = 83), followed by paraclinoid ICA (n = 50), PcomA (n = 21), anterior choroidal artery (AchoA) (n = 8), ophthalmic artery (n = 4), ICA bifurcation (n = 4), and one each at A1 or M1 arterial locations. Besides the three aneurysms with no baseline data, mean size of aneurysms (n = 341) was 5.2 ± 2.8 mm (range, 2.0–20.0 mm) and most of the aneurysms (325/341, 95.3%) measured ≤ 10 mm. Size ratios in paired aneurysms (169 patients) were as follows: 0–25%, 7 patients (4.1%); 26–50%, 36 patients (21.3%); 51–75%, 68 patients (40.2%); and 76–100%, 58 patients (34.4%). The size ratio in 126 patients (74.6%) was > 50%, indicating little size disparity in most of the paired lesions. Demographic data of this study population are summarized in Table 1.
Supplementary Table 1 (in the online-only Data Supplement) shows the demographic comparison of mirror aneurysms between patients with SAH and without SAH. The locations of the paired aneurysms were significantly different between both the groups (p = 0.01). In the unruptured group, paraclinoid ICA was the second most common location, whereas PcomA was the more common location in the ruptured group. In terms of rupture risk depending on the location, AchoA and PcomA were the locations with the highest risk of rupture (AchoA, 37.5%, PcomA, 28.6%; middle cerebral artery bifurcation, 9.6%; paraclinoid ICA, 2.0%; and others, 0%). Compared to the unruptured group, the D/N ratio of the ruptured group was larger (p < 0.01), whereas the size similarity and maximum diameter were not statistically significantly different between the two groups. However, in the ruptured group, the ruptured aneurysm had a larger size (7.62 ± 4.57 mm, p < 0.01) and a larger D/N ratio (1.52 ± 0.72, p = 0.26), as compared to the contralateral unruptured lesion (3.78 ± 1.47 mm, 1.20 ± 0.92, respectively).
Of the 344 aneurysms studied (in 172 patients), 217 were treated by coil embolization and 62 by surgical clipping. Observation alone (no treatment), with regular follow-up examinations, was advised for the remaining patients (n = 65), primarily due to low risk of rupture in small-sized or wide-necked and shallow aneurysms (n = 62). Branching arteries originated from aneurysmal domes in two patients; and given proximal tortuosity of parent artery in another, surgical clipping was advocated. However, this patient declined treatment.
Bilateral coil embolizations were performed in 83 patients, and 39 patients underwent unilateral coil embolizations along with regular observation of contralateral lesions. Similarly, bilateral surgical clipping was done in 12 patients, with unilateral clipping and contralateral observation in 26 patients. Concurrent coil embolization (one side) and surgical clipping (another side) were done in 12 patients. Bilateral coil embolizations were single-stage endeavors in 73 patients, with coiling done as separately staged procedures in 10 patients, either as an emergency intervention for rupture (n = 5) or during follow-up in instances of de novo development (n = 2), enlarging untreated lesions (n = 2), and contralateral aneurysmal rupture (n = 1). Eleven of the 12 patients who underwent bilateral surgical clipping also underwent separately staged procedures, with the primary treatment of lesions at higher risk. One patient presenting with SAH underwent single-stage bilateral clipping due to the ambiguity of the ruptured lesion (MCA bifurcation). Bilateral keyhole access was enabled through bicoronal skin incision.
In 18 patients presenting with SAH, ruptured aneurysms were treated in 16 (coiling, 11; clipping, 5), and dual lesions were simultaneously treated at acute phase in two patients (coiling, 1; clipping; 1).
Treatments elected are summarized in Table 2.
Of 217 aneurysms treated via coil embolization, complete occlusion was achieved in 38, with residual necks remaining in 150 and residual sacs persisting in 28. Coiling failed in one small-sized aneurysm, leaving only a stent deployed. Angiographic results were available in 40 of 62 patients subjected to surgical clipping. Total occlusion was achieved in 31 patients, remnant necks remained in eight, and a remnant sac persisted in one. The latter patient underwent a second coil embolization, without complications.
Follow-up imaging (≥ 6 months) was available in 213 of 217 aneurysms treated by coil embolization. The mean follow-up interval was 30.3 ± 22.0 months (range 6–177 months). During patient monitoring, 181 aneurysms showed complete occlusion, but recanalization was confirmed in 32 patients (major, 12; minor, 20). Repeat coil embolization was undertaken in all lesions showing major recanalization.
In 68 patients whose aneurysms were initially untreated (but monitored), follow-up imaging of 60 aneurysms was available (mean interval, 33.2 ± 21.3 months; range 4–92 months). While under observation, three of these lesions expanded in size and one was associated with SAH (Fig. 2). Two de novo aneurysms were also detected at paired sites in the aftermath of a treated unilateral aneurysm (Fig. 3). The five lesions above were treated by coil embolization. Overall, 65 patients with untreated aneurysms had undergone regular follow-up assessments.
At discharge, the functional status of four patients (2.3%) was poor (mRS ≥ 3). Three of them had suffered SAH of severe nature (Hunt-Hess grade 4), explaining the poor outcomes. The fourth patient (with unruptured aneurysm) developed venous infarction as a complication after surgical clipping and neurologic deficits ensued. At 6 months after discharge, only one patient (0.6%) remained functionally impaired (mRS of 4). The other three patients recovered fully, with no neurologic sequelae.
Single-stage bilateral coil embolizations of mirror aneurysms were done in 73 patients with 146 mirror aneurysms. In 13 patients, single-stage treatment included a third unpaired aneurysm. One patient presented with SAH. The most commonly employed endovascular technique in treating mirror aneurysms was stent assistance (n = 51), followed by single (n = 32) or multiple microcatheter (n = 30) use, balloon remodeling (n = 23), and microcatheter protection (n = 10). The pair of endovascular techniques is described in Supplementary Table 2 (in the online-only Data Supplement). Mean overall procedural time was 135.8 ± 40.9 minutes (range, 54–273 minutes). In patients with mirror aneurysms only, mean procedural time was 130.1 ± 37.6 minutes (range, 54–273 minutes), compared with 162.2 ± 46.6 minutes (range, 93–227 minutes) for mirror aneurysms with unpaired lesions (three aneurysms). Immediately after coil embolization, complete occlusion was achieved in 31, residual necks remained in 94, and residual sacs persisted in 20 patients. Coil insertion failed in one aneurysm, leaving only a stent deployed. Thromboembolic events occurred in three patients, which later resolved with intra-arterial tirofiban. All the thromboembolisms occurred in the case of stent assistance, and one of them had a bilateral stent (7.1%, 1/14). Coil migration and premature coil detachment developed in two patients, but the coils were retrieved via goose-neck snare, with no complications.
Radiologic imaging conducted after coil embolization (mean interval, 28.3 ± 18.6 months) in 72 patients showed complete occlusion in 125 aneurysms, minor recanalization in 11, and major recanalization in eight. There was no poor functional performance state (mRS ≥ 3) recorded at discharge or 6 months later.
The comparison between the single-stage and staged bilateral coil embolization in the mirror aneurysms is summarized in Table 3. In the single-stage bilateral coil embolization, the procedural time was shorter compared to staged embolization (p = 0.01), with the comparable result in terms of clinical and anatomical outcomes.
Mirror intracranial aneurysms occur in 2–12% of all patients with intracranial aneurysms (11121416). Despite their frequency of occurrence, they are not well studied. Only two large analyses in the past have focused on the sites of involvement and clinical characteristics (1112); however, a difference in the magnitude of mirror aneurysms has been noted in the reports. Meissner et al. (11) often found at least one sizeable lesion (> 10 mm in maximum diameter), more so than in non-mirror counterparts (53% vs. 42%, p = 0.007), with mirror aneurysms being larger than non-mirror aneurysms (11.7 mm vs. 10.4 mm; p < 0.001). According to Lee et al. (12), mirror aneurysms were comparatively smaller (< 5 mm) relative to single aneurysms (43.2% vs. 37.9%; p < 0.05). Most of the aneurysms (325/341, 95.3%) we studied measured ≤ 10 mm and 203 of 341 lesions (59.5%) measured < 5 mm. Although previous studies have compared mirror and non-mirror aneurysms in terms of size, paired mirror aneurysms have not been singularly compared. By nature, they are subject to the same systemic conditions and risk factors (i.e., age, sex, smoking status, alcohol consumption, and genetic variables) already known to influence their development and growth (4). Moreover, the symmetric origin of such lesions demonstrates similarities in hemodynamic stress and wall shear stress as etiologic forces (232425). For this reason, we speculated that mirror aneurysms might be of similar size, and indeed, lesions of 126 patients (74.6%) in our series showed little size disparity.
Earlier accounts have detailed the natural history of mirror aneurysms (1112), the management of such lesions is yet to be adequately investigated. According to Mehrotra et al. (16), surgical clipping conferred better neurologic outcomes in 17 ruptured mirror aneurysms, with less intraoperative rupture, vasospasm, and infarction than in multiple non-mirror aneurysms. Wang et al. (13) also studied patients with mirror aneurysms (n = 43), most of whom were treated by clipping (n = 39) rather than coil embolization (n = 4), and management of mirror aneurysms (clipping and coil embolization) has been detailed in some case series reports (1417181920). However, unlike the limited patient numbers amassed in prior efforts, this study included the largest sampling of treated mirror aneurysms to date. Of the 344 aneurysms in our series, coil embolization and surgical clipping were performed in 217 and 62 patients, respectively; and 65 aneurysms were observed without treatment.
In patients with multiple aneurysms presenting with SAH, distribution of hemorrhage and other features (large size, location, daughter sac, bottleneck factor, and height-width ratio) were evaluated to identify points of rupture (2627). Culprit lesions in patients with mirror aneurysms should be preferentially treated to avoid rebleeding, provided they can be reasonably distinguished. Of the 18 patients in our series who presented with SAH, singly ruptured lesions (exclusively) were treated at acute phase in 16 patients (coiling, 11; clipping, 5). The contralateral aneurysms were then addressed in separately staged procedures (coiling, 6; clipping, 2), except in eight patients whose untreated contralateral lesions were slated for observation. In this study, the ruptured lesion of the paired aneurysms had larger diameters and a higher D/N ratio than the contralateral unruptured aneurysm. In cases, when it is not possible to identify the ruptured lesion with certainty based on several imaging modalities, both the lesions should be treated in the acute phase. Dual lesions in two of our patients presenting with SAH were treated concurrently, one by single-stage bilateral coil embolization and the other through single-stage bilateral clipping. Wang et al. (28) also reported about the treatment strategies of a mirror aneurysm (a total of 70 pairs), in which the responsible ruptured aneurysm received treatment priority and the contralateral unruptured aneurysm was observed or treated in either single or multi-stage treatment. Therefore, emergent treatment of culprit lesion and staged management of the contralateral unruptured aneurysm are proposed as a rational treatment strategy of ruptured mirror aneurysm when the rupture lesion is evident.
Past attempts at single-stage surgical clipping of multiple aneurysms generally led to poor outcomes, due to excessive manipulation of cerebral arteries, the need for bilateral craniotomy, and prolonged brain retraction (2930). However, recent advances in microsurgery have enabled bilateral multipoint access by unilateral approach (31323334). Nevertheless, the narrow operative field exhibits difficulty in controlling intraoperative rupture and subsequently, the olfactory bulb rootlet becomes vulnerable to injury, and severe SAH (with brain swelling and diminished subfrontal corridor, limiting one-stage clipping) is a contraindication (31). In mirror aneurysms, dual clipping via unilateral approach is even more limited due to the distance between both the lesions. Compared with endovascular coiling, bilateral surgical clipping (requiring bilateral craniotomy) is more invasive, may take longer time, and may entail much more blood loss. If not technically prohibitive, single-stage coil embolization is thus preferred at our institution, rather than single-stage surgical clipping. A majority of patients (68.2%, 73/107) who elected bilateral intervention in this study underwent single-stage coil embolization.
In patients with unruptured mirror aneurysms, single-stage treatment of multiple aneurysms (if technically feasible) is advantageous to avoid second-round of general anesthesia, to eliminate the risk of rupture without delay, and to reduce hospitalization costs (35). Other reports have indicated that single-stage treatment is safe and effective in this setting (2836373839). Cho et al. (36) reported single-stage coil embolizations of 371 multiple aneurysms, performed in acceptable time intervals with few complications. The procedural time (111.5 ± 37.8 minutes), procedural morbidity (1 of 172, 0.6%), and post-procedural occlusion results (326 of 371, 87.9%) were comparable with our result. In another series of multiple aneurysms (n = 418) reported by Jeon et al. (39), morbidity and mortality of single-stage coiling were low. Wang et al. (28) successfully conducted 28 single-stage treatments of mirror aneurysms (clipping, 22; coil embolization, 8). In this study, the superiority of single-stage treatment over staged treatment could not be evaluated with regard to clinical outcome and complication rate because of the different indications applied to each group. However, in almost all the unruptured mirror aneurysms that were technically feasible, a single-stage coil embolization was performed without neurologic sequelae, and procedural time and anatomical outcomes were excellent. Therefore, single stage coil embolization is proposed as a viable option for the treatment of unruptured mirror aneurysms.
Our study has several limitations. First, entire data were collected retrospectively at a single institution, and criteria for treatment (modality and plan selection) were open to inconsistencies. In addition, patients electing treatment for at least one of two paired aneurysms were eligible for the study, so the range of baseline characteristics obtained may not accurately reflect all intracranial mirror aneurysms. Finally, aneurysms that were clipped were not routinely followed by imaging according to our institutional protocol. Therefore, long-term follow-up assessments were limited.
In conclusion, patients with mirror aneurysms can be safely and effectively treated by adapting therapeutic strategies to inherent configurations and vascular sources. If feasible, single-stage coil embolization should be considered as an excellent means of treating mirror aneurysms.
Figures and Tables
Fig. 2
Growth of untreated mirror aneurysms.
Unruptured mirror aneurysms (arrows) on MCAB: three dimensional images of right (A) and left MCAB (B); coil embolization of right MCAB aneurysm (C); and observation of left MCAB aneurysms; subarachnoid hemorrhage at 11 months after coil embolization (D); significant growth (arrow) of left MCAB aneurysm (E); and completion of angiography after coil embolization (F). MCAB = middle cerebral artery bifurcation
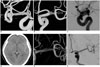
Fig. 3
De novo aneurysm at paired sites during follow-up after treatment of unilateral aneurysm.
Left PcomA aneurysm (arrow) on digital subtraction angiography at baseline (A); coil embolization of left PcomA aneurysm (B); de novo right PcomA aneurysm (arrow) seen at 36 months follow-up (C); and plain radiography after coil embolization (D). ICAG = internal carotid angiography, PcomA = posterior communicating artery
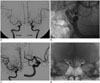
Table 1
Characteristics of Patients with Mirror Aneurysms

Table 2
Treatment Options of Mirror Aneurysms

Table 3
Comparison between Single-Stage and Staged Bilateral Coil Embolization in Mirror Aneurysms

References
1. Rinkel GJ. Natural history, epidemiology and screening of unruptured intracranial aneurysms. Rev Neurol (Paris). 2008; 164:781–786.

3. Brown RD Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014; 13:393–404.

4. Thompson BG, Brown RD Jr, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES Jr, et al. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015; 46:2368–2400.
5. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003; 362:103–110.

6. Lu HT, Tan HQ, Gu BX, Wu-Wang , Li MH. Risk factors for multiple intracranial aneurysms rupture: a retrospective study. Clin Neurol Neurosurg. 2013; 115:690–694.

7. Ellamushi HE, Grieve JP, Jäger HR, Kitchen ND. Risk factors for the formation of multiple intracranial aneurysms. J Neurosurg. 2001; 94:728–732.

9. Kaminogo M, Yonekura M, Shibata S. Incidence and outcome of multiple intracranial aneurysms in a defined population. Stroke. 2003; 34:16–21.

10. Nehls DG, Flom RA, Carter LP, Spetzler RF. Multiple intracranial aneurysms: determining the site of rupture. J Neurosurg. 1985; 63:342–348.

11. Meissner I, Torner J, Huston J 3rd, Rajput ML, Wiebers DO, Jones LK Jr, et al. International Study of Unruptured Intracranial Aneurysms Investigators. Mirror aneurysms: a reflection on natural history. J Neurosurg. 2012; 116:1238–1241.

12. Lee YJ, Parreira T, Matouk CC, Menezes R, Mandell DM, terBrugge KG, et al. Clinical characteristics and preferential location of intracranial mirror aneurysms: a comparison with non-mirror multiple and single aneurysms. Neuroradiology. 2015; 57:35–40.

13. Wang R, Zhang D, Zhao J, Wang S, Zhao Y, Niu H. A comparative study of 43 patients with mirror-like intracranial aneurysms: risk factors, treatment, and prognosis. Neuropsychiatr Dis Treat. 2014; 10:2231–2237.
14. Baccin CE, Krings T, Alvarez H, Ozanne A, Lasjaunias P. Multiple mirror-like intracranial aneurysms. Report of a case and review of the literature. Acta Neurochir (Wien). 2006; 148:1091–1095. discussion 1095.

15. Casimiro MV, McEvoy AW, Watkins LD, Kitchen ND. A comparison of risk factors in the etiology of mirror and nonmirror multiple intracranial aneurysms. Surg Neurol. 2004; 61:541–545.

16. Mehrotra A, Sharma P, Das KK, Bhaisora KS, Sardhara J, Gupta S, et al. Mirror aneurysms among multiple aneurysms: lesser of the two evils. World Neurosurg. 2016; 92:126–132.

17. Lesley WS. Bilateral mirror posterior inferior cerebellar artery aneurysms: diagnostic caveat on catheter angiography. J Neurointerv Surg. 2011; 3:383–385.

18. Xu Y, Chen SD, Lei B, Zhang WH, Wang WY. One-stage operation for rare multiple mirror intracranial aneurysms: a case report and literature review. Turk Neurosurg. 2014; 24:598–601.
19. Enesi E, Rroji A, Demneri M, Vreto G, Petrela M. Mirror image distal anterior cerebral artery aneurysms treated with coil embolization. a report of two cases and literature review. Interv Neuroradiol. 2013; 19:49–55.
20. Yamada K, Nakahara T, Kishida K, Yano T, Yamamoto K, Ushio Y. Multiple “mirror” aneurysms involving intracavernous carotid arteries and vertebral arteries: case report. Surg Neurol. 2000; 54:361–365.

21. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003; 34:1398–1403.

22. Jeon JP, Cho YD, Rhim JK, Park JJ, Cho WS, Kang HS, et al. Stent-assisted coil embolization of vertebrobasilar dissecting aneurysms: procedural outcomes and factors for recanalization. Korean J Radiol. 2016; 17:801–810.

23. Feng Y, Wada S, Tsubota K, Yamaguchi T. The application of computer simulation in the genesis and development of intracranial aneurysms. Technol Health Care. 2005; 13:281–291.

24. Wang F, Xu B, Sun Z, Wu C, Zhang X. Wall shear stress in intracranial aneurysms and adjacent arteries. Neural Regen Res. 2013; 8:1007–1015.
25. Munarriz PM, Gómez PA, Paredes I, Castaño-Leon AM, Cepeda S, Lagares A. Basic principles of hemodynamics and cerebral aneurysms. World Neurosurg. 2016; 88:311–319.

26. Hoh BL, Sistrom CL, Firment CS, Fautheree GL, Velat GJ, Whiting JH, et al. Bottleneck factor and height-width ratio: association with ruptured aneurysms in patients with multiple cerebral aneurysms. Neurosurgery. 2007; 61:716–722. discussion 722-723.
27. Baumann F, Khan N, Yonekawa Y. Patient and aneurysm characteristics in multiple intracranial aneurysms. Acta Neurochir Suppl. 2008; 103:19–28.

28. Wang WX, Xue Z, Li L, Wu C, Zhang YY, Lou X, et al. Treatment strategies for intracranial mirror aneurysms. World Neurosurg. 2017; 100:450–458.

29. Rinne J, Hernesniemi J, Niskanen M, Vapalahti M. Management outcome for multiple intracranial aneurysms. Neurosurgery. 1995; 36:31–37. discussion 37-38.

30. Mizoi K, Suzuki J, Yoshimoto T. Surgical treatment of multiple aneurysms. Review of experience with 372 cases. Acta Neurochir (Wien). 1989; 96:8–14.
31. Rodríguez-Hernández A, Gabarrós A, Lawton MT. Contralateral clipping of middle cerebral artery aneurysms: rationale, indications, and surgical technique. Neurosurgery. 2012; 71:1 Suppl Operative. 116–123. discussion 123-124.
32. Wachter D, Kreitschmann-Andermahr I, Gilsbach JM, Rohde V. Early surgery of multiple versus single aneurysms after subarachnoid hemorrhage: an increased risk for cerebral vasospasm? J Neurosurg. 2011; 114:935–941.

33. Hong T, Wang Y. Unilateral approach to clip bilateral multiple intracranial aneurysms. Surg Neurol. 2009; 72:Suppl 1. S23–S28. discussion S28.

34. Santana Pereira RS, Casulari LA. Surgical treatment of bilateral multiple intracranial aneurysms. Review of a personal experience in 69 cases. J Neurosurg Sci. 2006; 50:1–8.
35. Xavier AR, Rayes M, Pandey P, Tiwari A, Kansara A, Guthikonda M. The safety and efficacy of coiling multiple aneurysms in the same session. J Neurointerv Surg. 2012; 4:27–30.

36. Cho YD, Ahn JH, Jung SC, Kim CH, Cho WS, Kang HS, et al. Single-stage coil embolization of multiple intracranial aneurysms: technical feasibility and clinical outcomes. Clin Neuroradiol. 2016; 26:285–290.

37. Andic C, Aydemir F, Kardes O, Gedikoglu M, Akin S. Single-stage endovascular treatment of multiple intracranial aneurysms with combined endovascular techniques: is it safe to treat all at once? J Neurointerv Surg. 2017; 9:1069–1074.

Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3348/kjr.2018.19.5.849.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download