Abstract
Objective
Several studies have demonstrated the neuroprotective effects of (+)-MK-801 hydrogen maleate (dizocilpine), in various animal models of hypoxic-ischemic (HI) brain injury. However limited data are available on the neonatal model of HI brain injury. The aim of the present study was to investigate the effects of dizocilpine and its mechanisms associated with NMDARs expression in neonatal rat model of HI brain injury.
Methods
In in vivo model, 7d-old rat pups underwent permanent unilateral carotid ligation. The animals were divided into six groups: N, normoxia; H, hypoxia without operation; HS, hypoxia with Sham operation; HO, hypoxia with operation; HV, HO treated with vehicle; HD, HO treated with dizocilpine. Dizocilpine (10 mg/kg) was administered intracerebrally to the rats 30 min before HI brain injury. Rat pups were exposed to hypoxia by placing them for 2 hours in hypoxic incubator (92% N2, 8% O2). In in vitro model, embryonic cortical neuronal cell cultures (from SD rats of embryonic days of 18) were done. The normoxia (N) group was prepared in 5% CO2 incubators. The hypoxia (H), and hypoxia treated with dizocilpine (HD) groups were placed in 1% O2 incubators (94% N2, 5% CO2) for 16 hours. In order to estimation of cell viability and growth, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay was done. The degree of neuronal death was evaluated by morphometric method and the protein expression of each NMDARs was quantified by Real Time-PCR and Western blot.
Results
Both in thein vitro andin vivo models, the expressions of NMDAR subunits were lower in the hypoxia group than in the normoxia group, whereas they increased in the hypoxia treated with dizocilpine group compared to the hypoxia group. In vitro model, however, the expressions of NR1, NR2A mRNAs decreased in the H group when compared to the N group, whereas they increased a little in the HD group when compared to the H group.
REFERENCES
1.Monaghan DT., Bridges RJ., Cotman CW. The excitatory amino acid receptors: Their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989. 29:365–402.

2.Roberts EL., Wisotzky D., Chih CP. Aging and the effects of MK-801 on anoxic damage in rat hippocampal slices. Brain research. 1998. 791:321–4.

3.Jang YJ., Seo ES., Kim WT. Neuroprotection of Recombinant Human Erythropoietin Via Modulation of N-methyl-D-aspartate Receptors in Neonatal Rats with Hypoxic-ischemic Brain Injury. J Korean Soc Neonatol. 2009. 16:221–33.
4.Go HY., Seo ES., Kim WT. Expression of nitric oxide synthase isoforms and N-methyl-D-aspartate receptor subunits according to transforming growth factor-beta1 administration after hypoxic-ischemic brain injury in neonatal rats. Korean J Pediatr. 2009. 52:594–602.
5.Mishra OP., Delivoria-Papadopoulos M. NMDA receptor modification of the fetal guinea pig brain during hypoxia. Neurochem Res. 1992. 17:1211–6.
6.Park CK., Nehls DG., Graham DI., Teasdale GM., McCulloch J. The glutamate antagonist MK-801 reduces focal ischemic brain damage in the rat. Ann Neurol. 1988. 24:543–51.

7.Gilland E., Hagberg H. NMDA-receptor dependent increase of cerebral glucose utilization after hypoxia-ischemia in the neonatal rat. J Cereb Blood Flow Metab. 1996. 16:1005–13.
8.Seo MA., Lee HJ., Choi EJ., Kim JK., Chung HL., Kim WT. Neuroprotective Effect of Dizocilpine (MK-801) via Anti-apoptosis on Hypoxic-ischemic Brain Injury in Neonatal Rats. J Korean Soc Neonatol. 2010. 17:181–92.

9.Jo JY., Lee HJ., Choi EJ., Kim JK., Chung HL., Kim WT. Neuroprotective Effects of Dizocilpine (MK-801) Via Mediation of Nitric Oxide Synthase on Hypoxic-ischemic Brain Injury in Neonatal Rats. Korean J Perinatol. 2011. 22:181–93.
10.Alilain WJ., Goshgarian HG. MK-801 upregulates NR2A protein levels and induces functional recovery of the ipsilateral hemidiaphragm following acute C2 hemisection in adult rats. J Spinal Cord Med. 2007. 30:346–54.

11.Almirón RS., Pérez MF., Ramírez OA. MK-801 prevents the increased NMDA-NR1 and NR2B subunits mRNA expression observed in the hippocampus of rats tolerant to diazepam. Brain Res. 2004. 1008:54–60.

12.Feng Y., Fratkin JD., LeBlanc MH. Treatment with tamoxifen reduces hypoxic-ischemic brain injury in neonatal rats. Eur J Pharmacol. 2004. 484:65–74.

13.Rice JE., Vannucci RC., Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981. 9:131–41.

14.Brewer GJ. Isolation and culture of adult rat hippocampal neurons. J Neurosci Methods. 1997. 71:143–55.

15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65:55–63.

16.Mishra OP., Fritz KI., Delivoria-Papadopoulos M. NMDA receptor and neonatal hypoxic brain injury. Ment Retard Dev Disabil Res Rev. 2001. 7:249–53.

19.Romijn HJ., Hofman MA., Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term human baby? Early Hum Dev. 1991. 26:61–7.
20.Jeong JE., Kim TY., Park HJ., Lee KH., Choi EJ, et al. Taurine exerts neuroprotective effects via anti-apoptosis in hypoxic-ischemic brain injury in neonatal rats. Korean J Pediatr. 2009. 52:1337–47.

21.Ford LM., Sanberg PR., Norman AB., Fogelson MH. MK-801 prevents hippocampal neurodegeneration in neonatal hypoxic-ischemic rats. Arch Neurol. 1989. 46:1090–6.

22.Hossmann KA. Periinfarct depolarizations. Cerebrovasc Brain Metab Rev. 1996. 8:195–208.
23.Sugihara H., Moriyoshi K., Ishii T., Masu M., Nakanishi S. Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem Biophys Res Commun. 1992. 185:826–32.

24.Meguro H., Mori H., Araki K., Kushiya E., Kutsuwada T., Yamazaki M, et al. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992. 357:70–4.

25.Gurd JW., Bissoon N., Beesley PW., Nakazawa T., Yamamoto T., Vannucci SJ. Differential effects of hypoxia-ischemia on subunit expression and tyrosine phosphorylation of the NMDA receptor in 7- and 21-day-old rats. J Neurochem. 2002. 82:848–56.

26.Hsu JC., Zhang Y., Takagi N., Gurd JW., Wallace MC., Zhang L, et al. Decreased expression and functionality of NMDA receptor complexes persist in the CA1, but not in the dentate gyrus after transient cerebral ischemia. J Cereb Blood Flow Metab. 1998. 18:768–75.

27.Band M., Malik A., Joel A., Avivi A. Hypoxia associated NMDA receptor 2 subunit composition: developmental comparison between the hypoxia-tolerant subterranean mole-rat, Spalax, and the hypoxia-sensitive rat. J Comp Physiol B. 2012. 182:961–9.

28.Bickler PE., Fahlman CS., Taylor DM. Oxygen sensitivity of NMDA receptors: relationship to NR2 subunit composition and hypoxia tolerance of neonatal neurons. Neuroscience. 2003. 118:25–35.

29.Johnson EM Jr., Deckwerth TL. Molecular mechanisms of developmental neuronal death. Annu Rev Neurosci. 1993. 16:31–46.

30.Dubinsky JM. Intracellular calcium levels during the period of delayed excitotoxicity. J Neurosci. 1993. 13:623–31.

31.Manev H., Favaron M., Guidotti A., Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol Pharmacol. 1989. 36:106–12.
32.Wood PL., Rao TS., Iyengar S., Lanthorn T., Monahan J., Corki A, et al. A review of thein vitro andin vivo neurochemical characterization of the NMDA/PCP/glycine/ion channel receptor macrocomplex. Neurochem Res. 1990. 15:217–30.
33.Goda M., Isono M., Fujiki M., Kobayashi H. Both MK801 and NBQX reduce the neuronal damage after impact-acceleration brain injury. J Neurotrauma. 2002. 19:1445–56.

34.Spandou E., Karkavelas G., Soubasi V., Avgovstides-Savvo-poulou P., Loizidis T., Guiba-Tziampiri O. Effect of ketamine on hypoxic-ischemic brain damage in newborn rats. Brain Res. 1999. 819:1–7.

35.Gómez-Pinilla F., Tram H., Cotman CW., Nieto-Sampedro M. Neuroprotective effect of MK-801 and U-50488H after contusive spinal cord injury. Exp Neurol. 1989. 104:118–24.
Fig. 1.
Western blots of NR2A (A; N, 100±2.1; H, 107.5±2.1; HS, 103.2±2.1; HO, 85.1±1.7; HV, 90.5±1.8; HD, 98.5±1.9) and NR2B (B; N, 100±2.0; H, 101.6±2.0; HS, 101.2±2.0; HO, 87.9±1.8; HV, 82.3±1.6; HD, 102.6± 2.1) in the neonatal hypoxic-ischemic brain injury (in vivo) (n=4). Dizocilpine was administered at 10 mg/kg. N, normoxia; H, hypoxia without operation; HS, hypoxia with Sham operation; HO, hypoxia with operation; HV, HO treated with vehicle; HD, HO treated with dizocilpine; *P<0.05, statistically significant vs. HO.
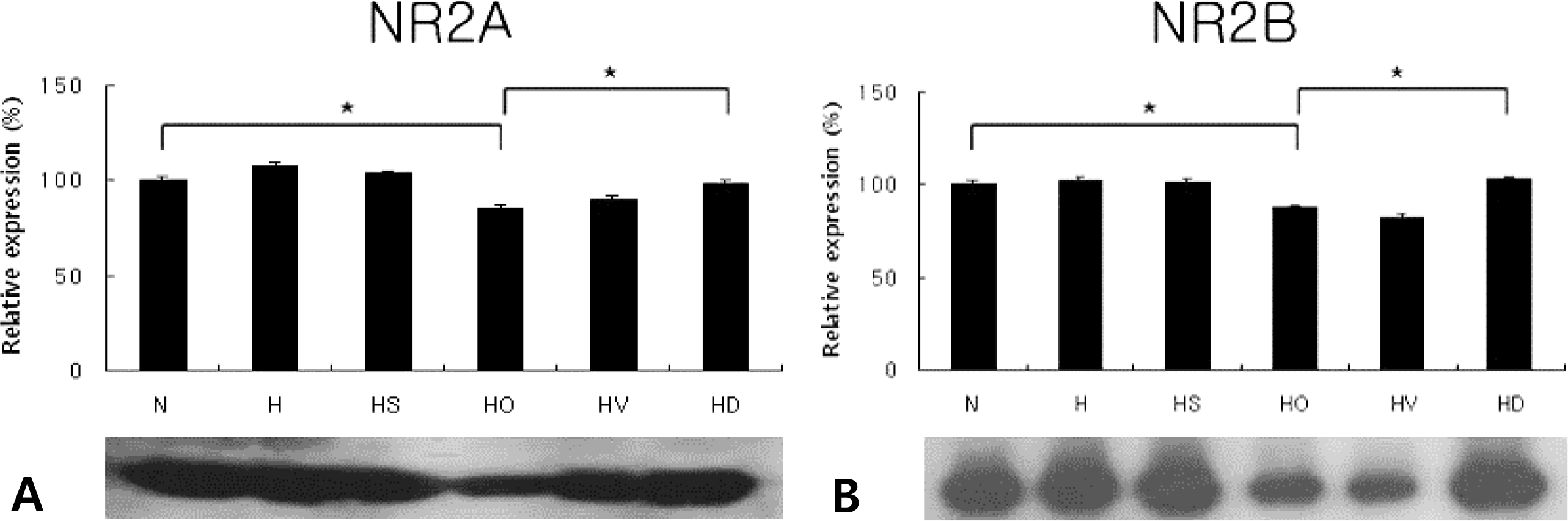
Fig. 2.
Real-time PCRs of NR1 (A; N, 100±3.0; H, 125.7±3.8; HS, 104.9±3.1; HO, 36.1±1.1; HV, 62.9±1.8; HD, 119.7±3.4), NR2A (B; N, 100±3.1; H, 137.6±4.1; HS, 123.1±3.6; HO, 43.8±1.3; HV, 41.8±1.3; HD, 85.9±2.6), NR2B (C; N, 100±3.3; H, 127.5±3.8; HS, 100.7±3.0; HO, 66.9±2.0; HV, 58.6±1.8; HD, 80.7±2.4), NR2C (D; N, 100±3.1; H, 112.5±3.4; HS, 89.5±2.6; HO, 42.3±1.3; HV, 47.9±1.4; HD, 56.6±1.7), and NR2D (E; N, 100±3.1; H, 104.2±3.1; HS, 91.4±2.7; HO, 46.0±1.3; HV, 47.9±1.4; HD, 81.8±2.4) mRNAs in the neonatal hypoxic-ischemic brain injury (in vivo) (n=6). Dizocilpine was administered at 10 mg/kg. N, normoxia; H, hypoxia without operation; HS, hypoxia with Sham operation; HO, hypoxia with operation; HV, HO treated with vehicle; HD, HO treated with dizocilpine; *P<0.05, statistically significant vs. HO.
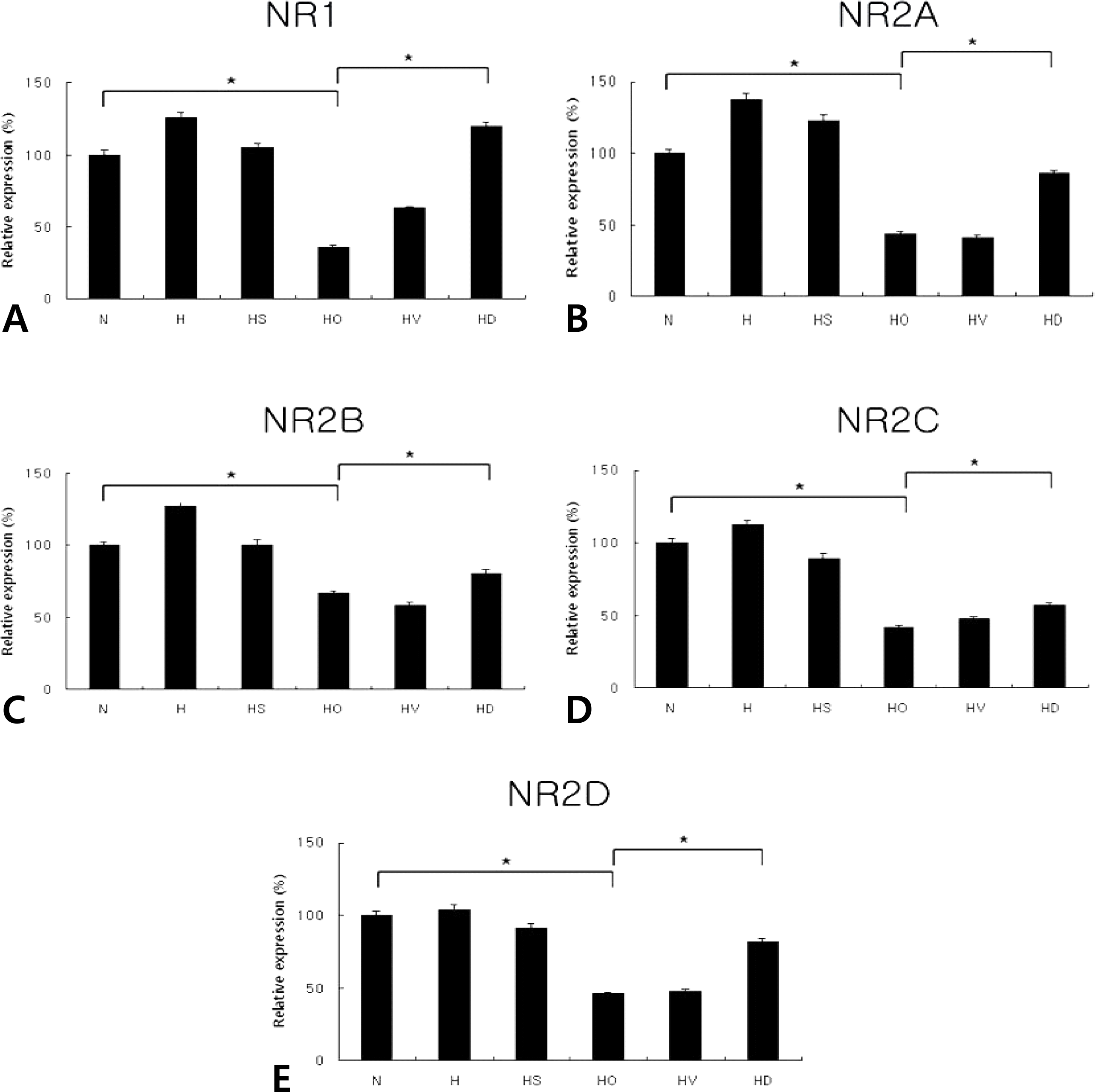
Fig. 3.
Morphologic changes in the embryonic cortical neuronal cell culture of rat (in vitro). A, normoxia; B, hypoxia; C, hypoxia treated with dizocilpine.
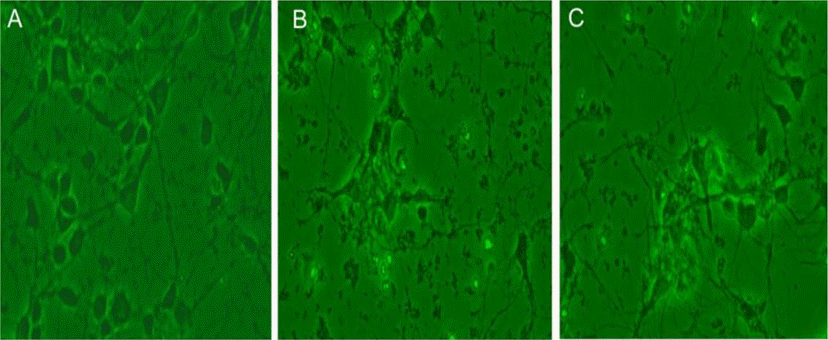
Fig. 4.
Cell viability was measured by the 3-(4,5-dimethylthi-azol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Dizocilpine was administered at 10 μg/mL. N, normoxia; H, hypoxia; HD, hypoxia treated with dizocilpine; *P<0.05, statistically significant vs. H.
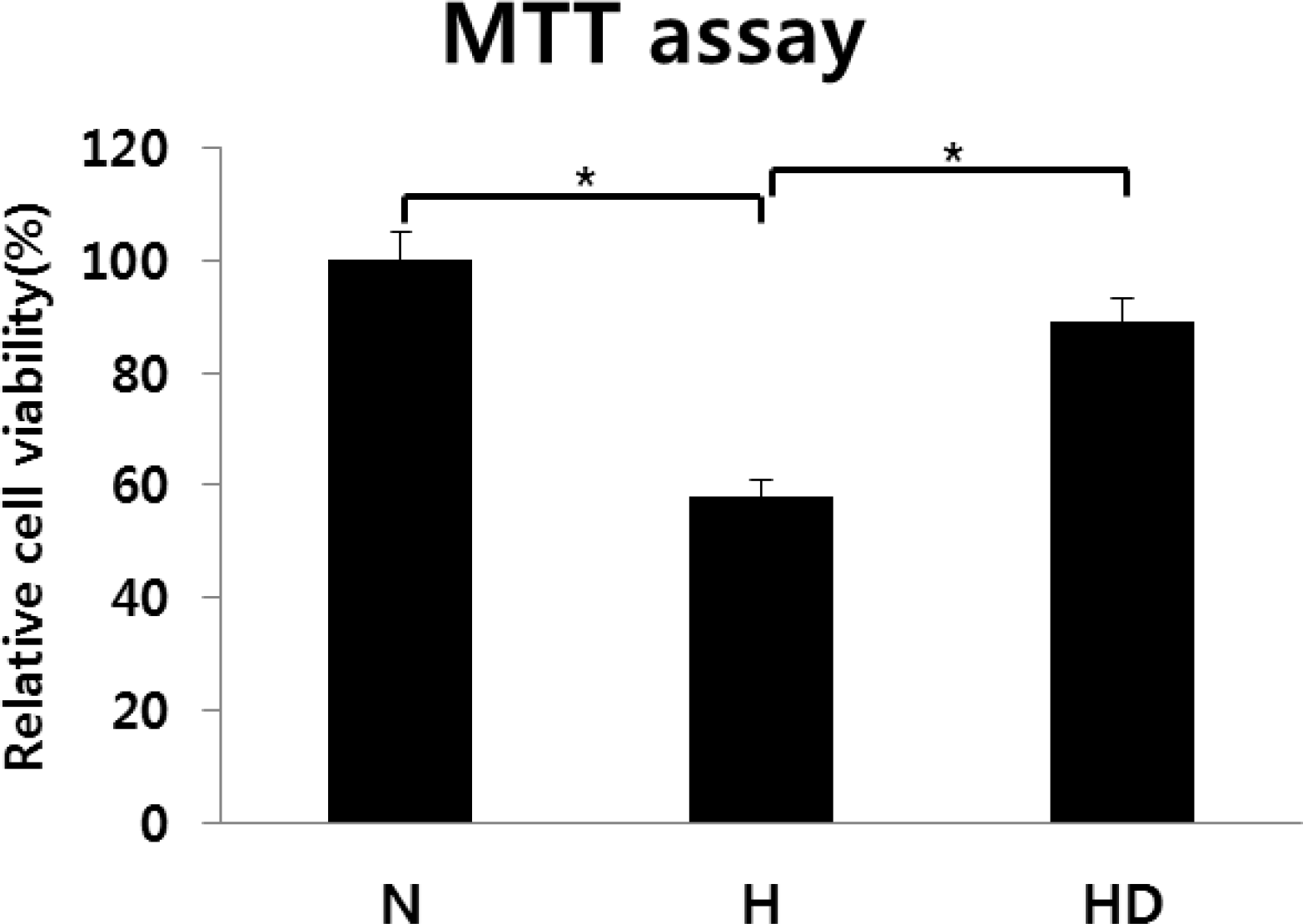
Fig. 5.
Western blots (A) of NR2A (B; N, 100±2.1; H, 52.5±1.5; HD, 67.1±1.6) and NR2B (C; N, 100± 2.1; H, 89.4±1.8; HD, 100.5±2.0) in the embryonic cortical neuronal cell culture (in vitro) (n=4). Dizocilpine was administered at 10 μg/mL. N, normoxia; H, hypoxia; HD, hypoxia treated with dizocilpine; *P<0.05, statistically significant vs. H.
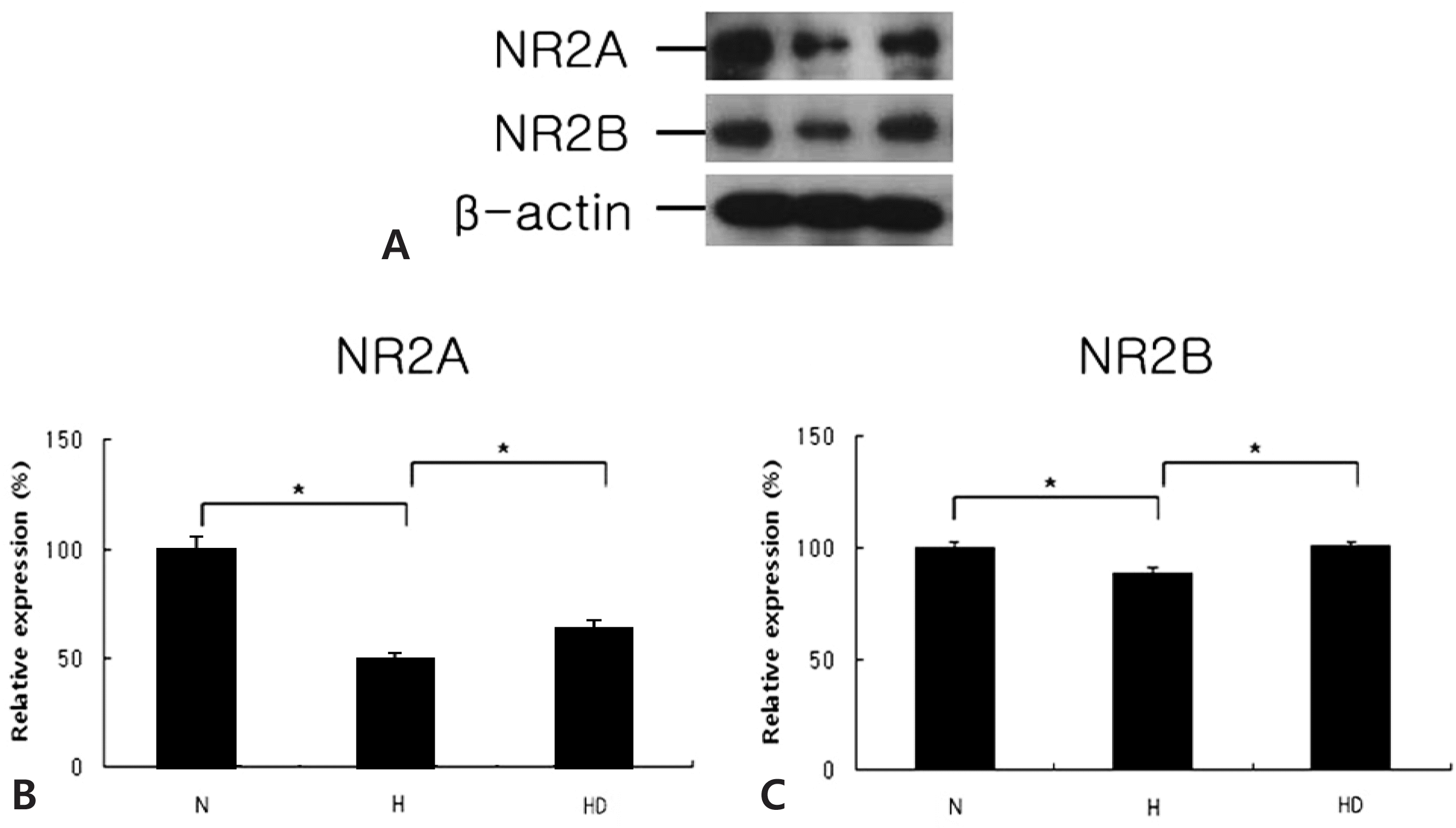
Fig. 6.
Real-time PCRs of NR1 (A; N, 100±4.1; H, 34.0±1.4; HD, 39.5±1.6), NR2A (B; N, 100±4.0; H, 28.7±1.1; HD, 34.2±1.4), NR2B (C; N, 100±4.0; H, 47.5±1.9; HD, 56.1±2.2), NR2C (D; N, 100±4.2; H, 19.8±0.8; HD, 36.1±1.4), and NR2D (E; N, 100±4.1; H, 56.3±2.3; HD, 117.7±4.7) in the embryonic cortical neuronal cell culture (in vitro) (n=6). Dizocilpine was administered at 10 μg/mL. N, normoxia; H, hypoxia; HD, hypoxia treated with dizocilpine; *P<0.05, statistically significant vs. H.
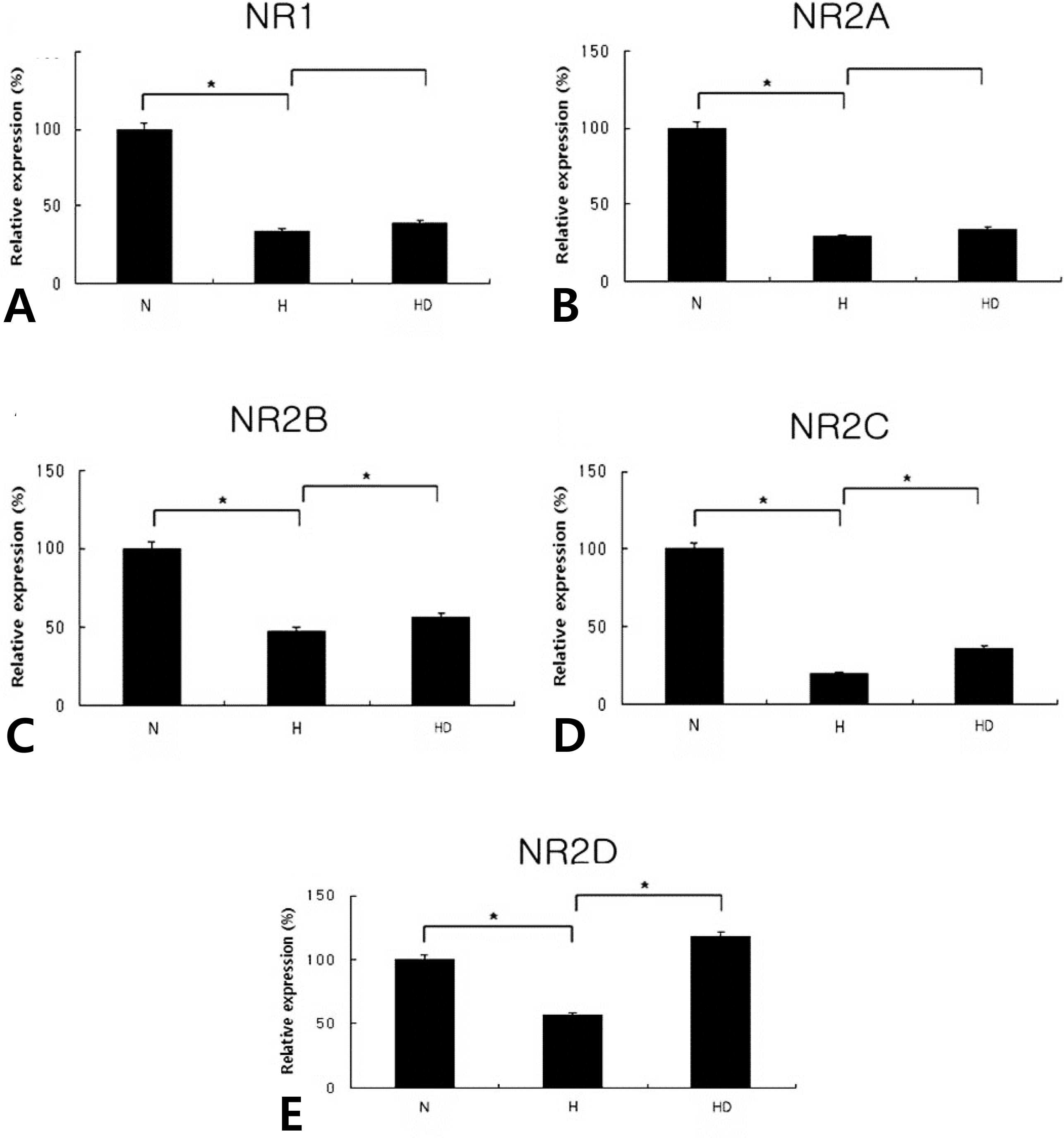
Table 1.
Primer Pairs and Annealing Temperature for Realtime PCR




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download