Abstract
Background
We attempted to determine the characteristics of diarrheal pathogens according to species, seasonal variations, and patient age using multiplex PCR for the epidemiologic study of diarrheal disease in Jeju Island.
Methods
From March 2015 to Feb 2017, stool specimens were collected from 537 diarrheal patients older than 16 years. Multiplex PCR was used to identify pathogens and found Group A Rotavirus, enteric Adenovirus, Norovirus GI/GII, Astrovirus, Salmonella spp., Shigella spp., Vibrio spp., Campylobacter spp., Clostridium difficile toxin B (CDB), Clostridium perfringens, Yersinia enterocolitica, Aeromonas spp., Escherichia coli O157:H7, and verocytotoxin-producing E. coli (VTEC).
Results
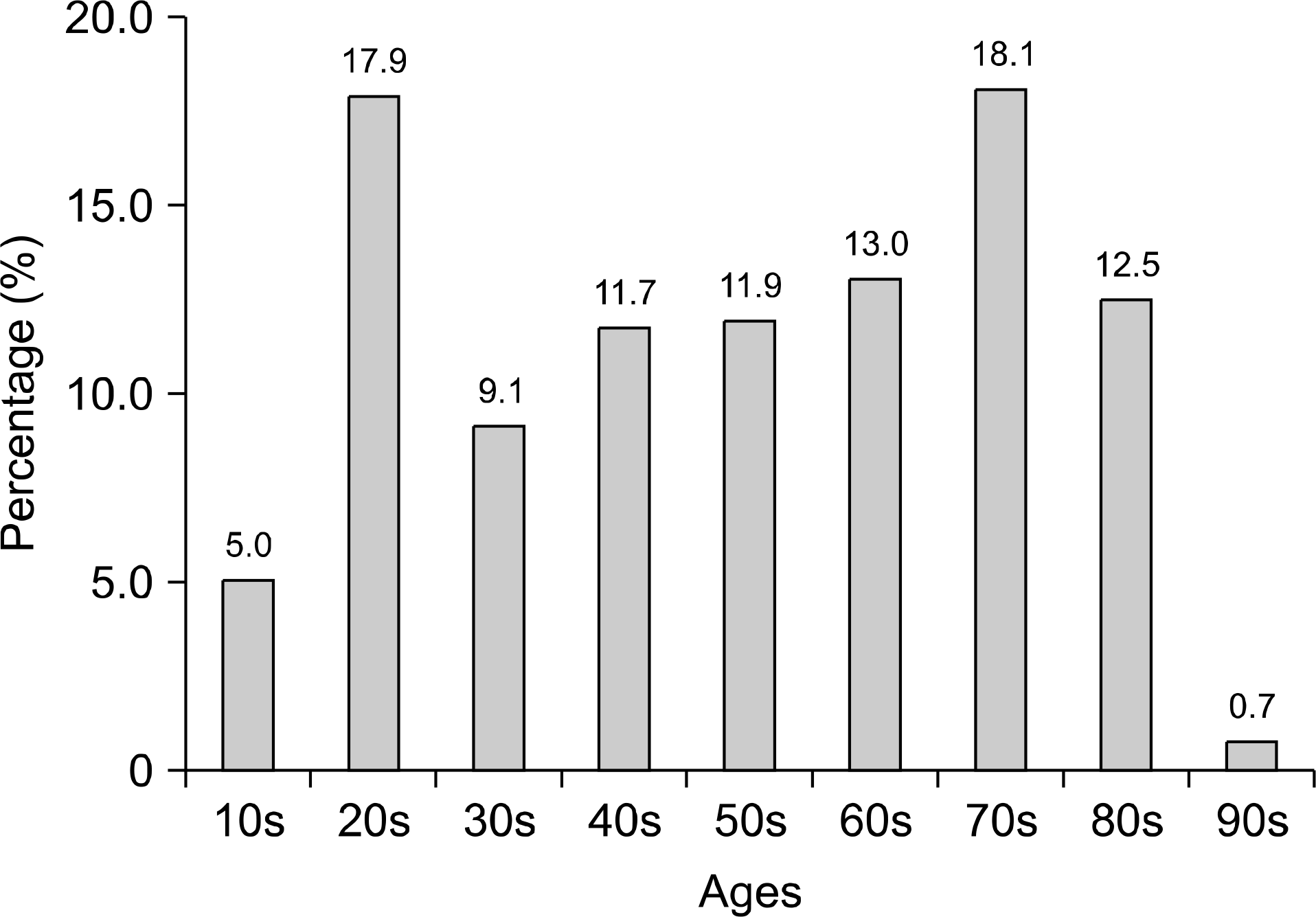
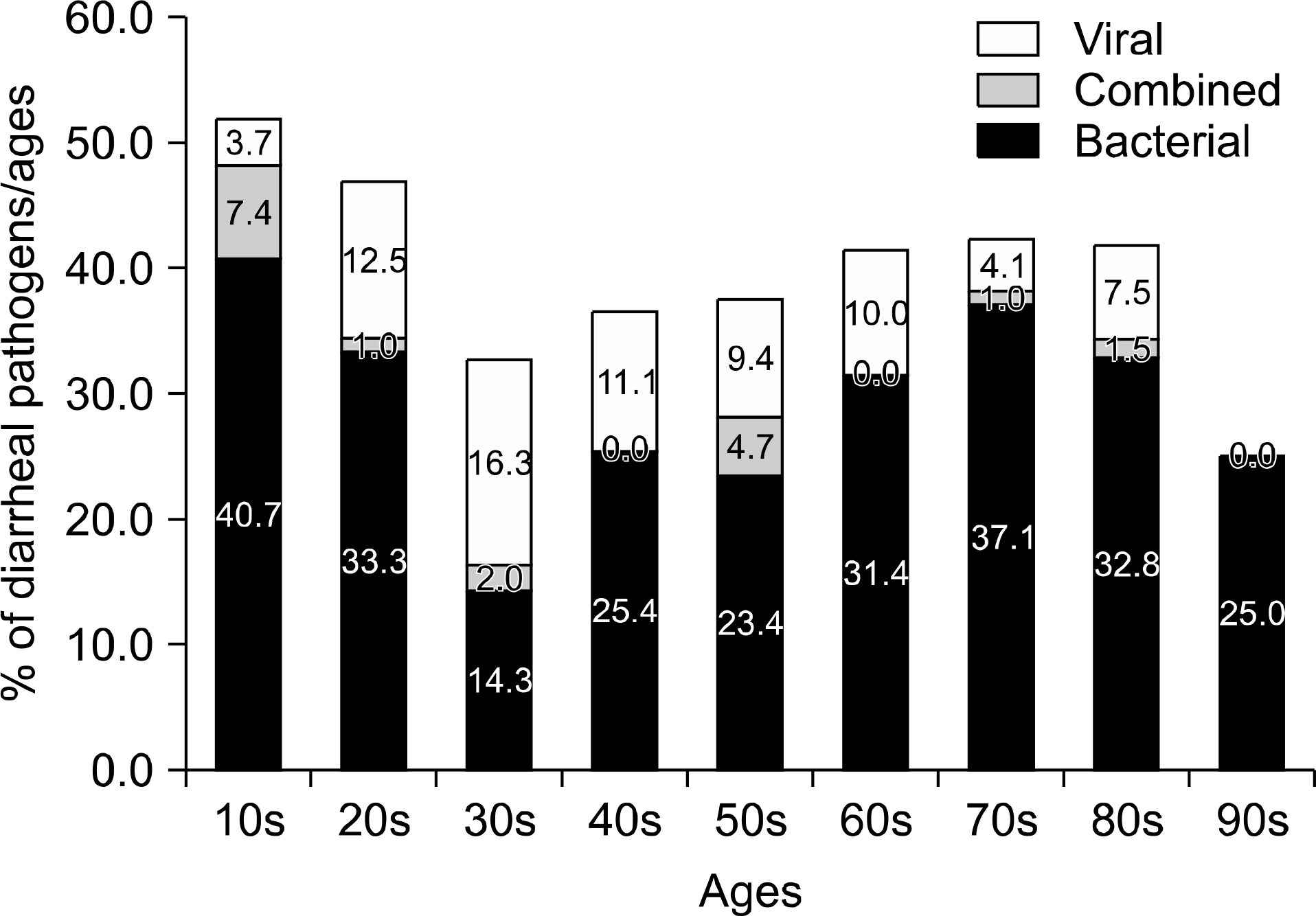
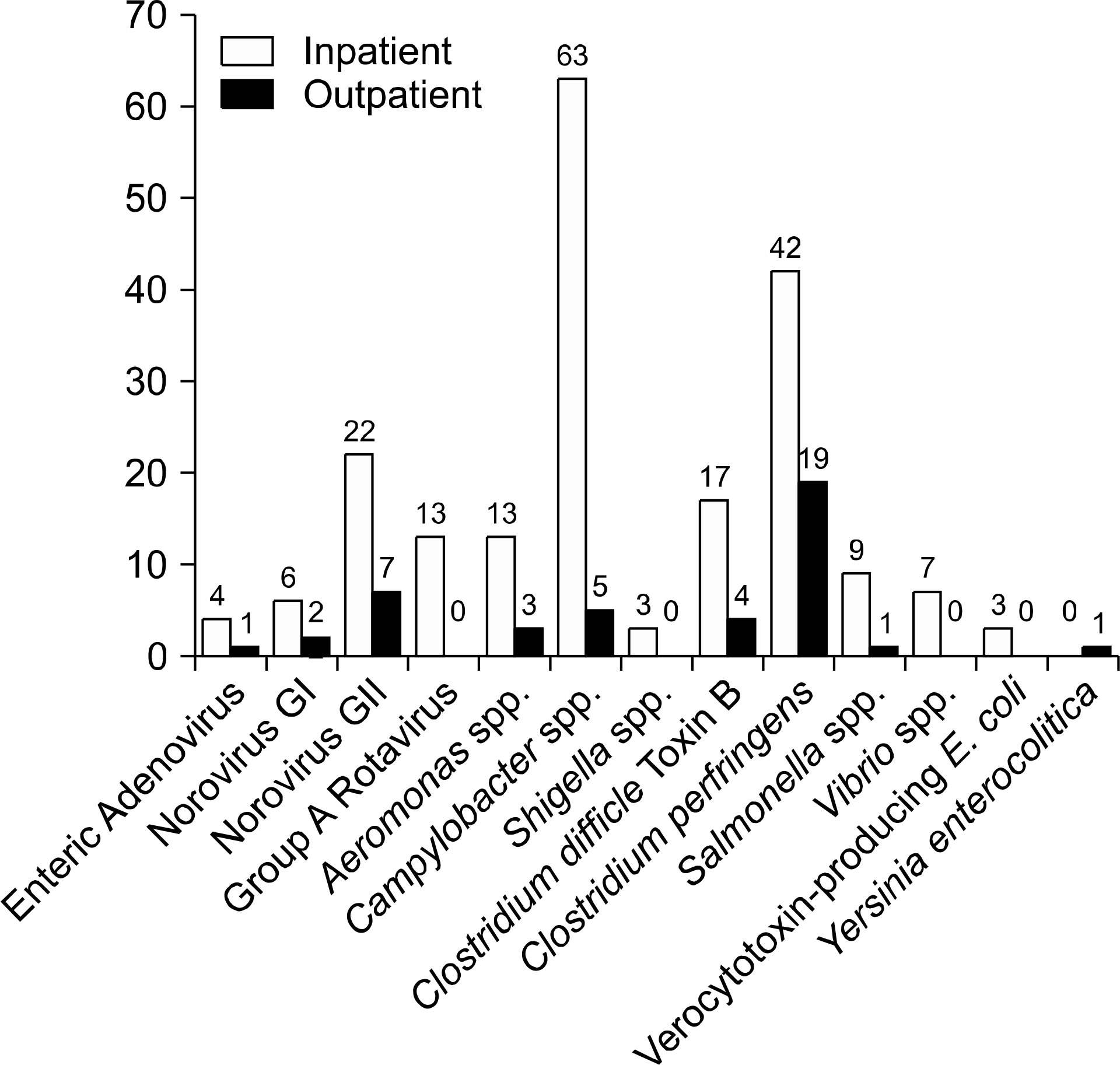
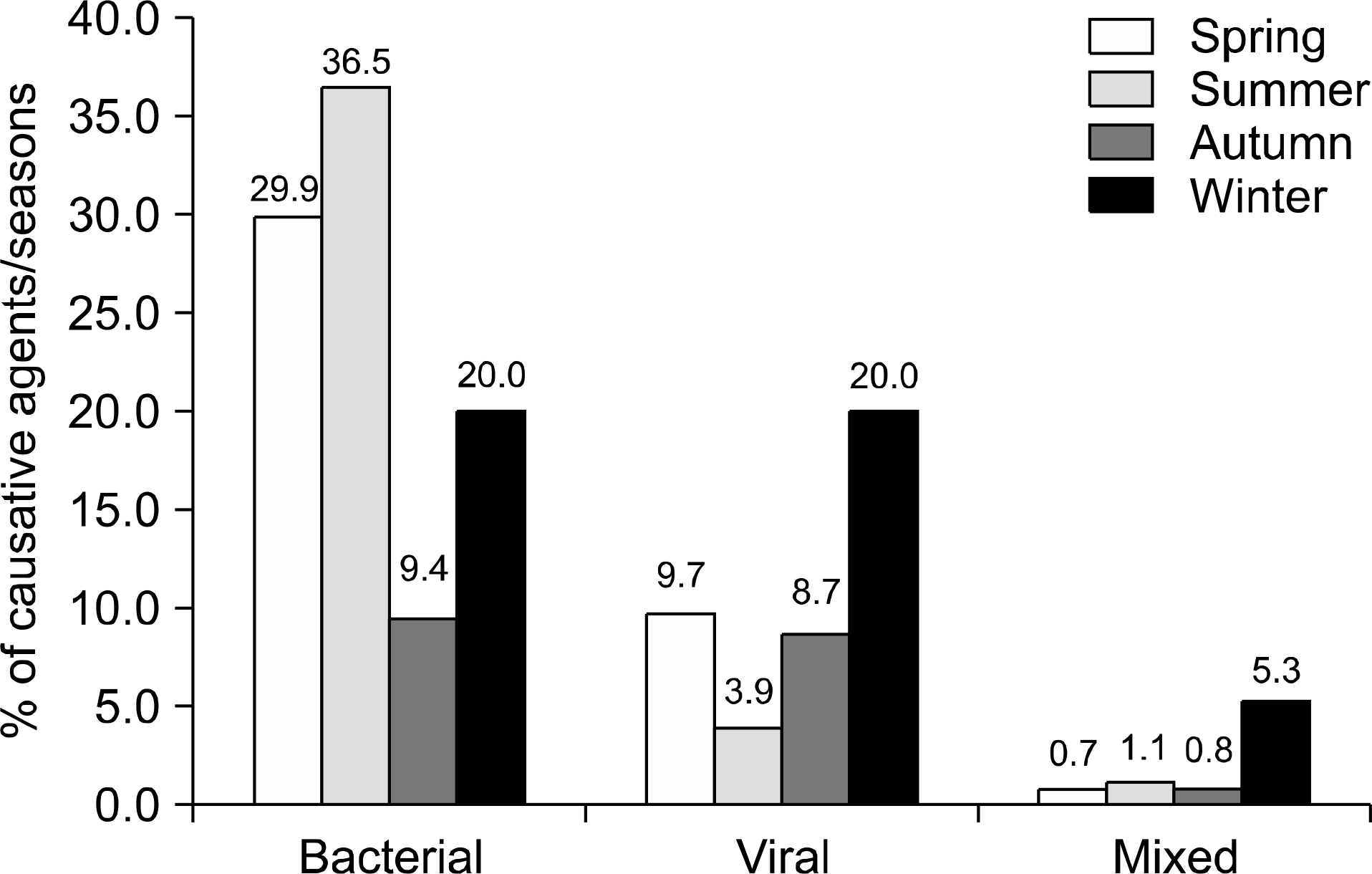
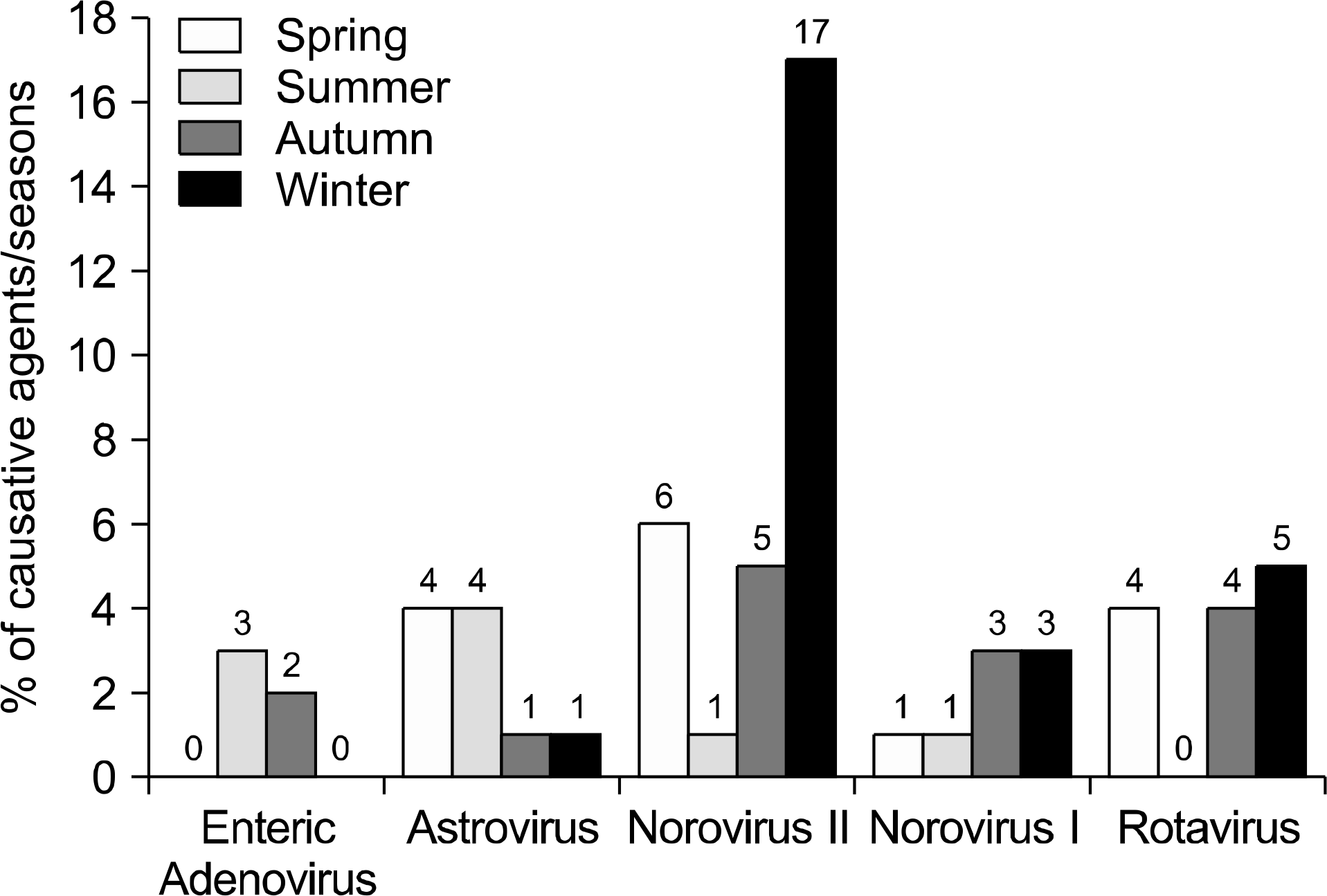
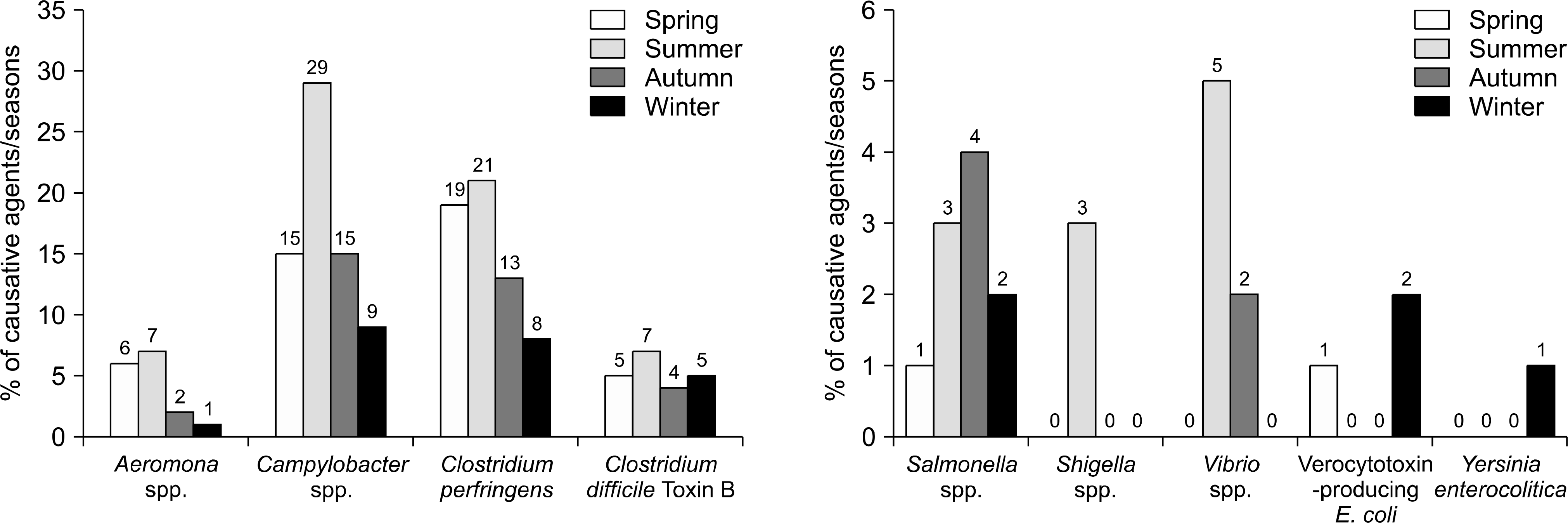
Pathogens were isolated from 221 of 537 samples (41.2%); 9.3% were positive only for viral pathogens; 30.2%, only for bacterial pathogens; and 1.7%, for both viral and bacterial pathogens. Bacteria were more prevalent in spring, summer, and autumn, but viral pathogens were more prevalent in winter. Overall prevalence were Campylobacter spp. (26.7%), Clostridium perfringens (23.9%); Norovirus GII (11.4%), CDB (8.2%), Aeromonas spp. (6.3%), Group A Rotavirus (5.1%), Salmonella spp. (3.9%), Astrovirus (3.9%), Norovirus GI (3.1%), Vibrio spp (2.7%), enteric Adenovirus (1.6%), Shigella spp. (1.2%), VTEC (1.2%), and Yersinia enterocolitica (0.4%). Group A Rotavirus and Norovirus GII were more prevalent in winter and early spring. Campylobacter spp., CDB, and C. perfringens were detected frequently, without seasonal variation.
Conclusion
Bacterial pathogens are more prevalent than viruses in acute diarrhea in adults living in Jeju Island, especially in spring, summer, and autumn. Viral pathogens are prevalent in winter. Campylobacter spp., CDB, and Clostridium perfringens are the major pathogens occurring without seasonal variations. These data will be helpful in identifying diarrheal pathogens and for treatments and prevention strategies.
Go to : 
References
1. Pai H. Acute infectious diarrhea. Korean J Med. 2007; 73:114–8.
2. Lee S, Park YJ, Lee HK, Kim SY, Kim JY, Lee SY, et al. Detection of 13 enteric bacteria and 5 viruses causing acute infectious diarrhea using multiplex PCR from direct stool specimens. Ann Clin Microbiol. 2013; 16:33–8.

3. Kim NO, Cha I, Kim JS, Chung GT, Kang YH, Hong S. The prevalence and characteristics of bacteria causing acute diarrhea in Korea, 2012. Ann Clin Microbiol. 2013; 16:174–81.

4. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016; 111:602–22.

5. Choi WH, Byun JH, Kim S. Prevalence of bacteria in the nationwide survey of stool culture performed in 2015, Korea. Ann Clin Microbiol. 2016; 19:105–9.

6. Ma SH. Acute infectious diarrhea in pediatirc patients. Korean J Pediatr. 2005; 48:235–50.
7. Chikhi-Brachet R, Bon F, Toubiana L, Pothier P, Nicolas JC, Flahault A, et al. Virus diversity in a winter epidemic of acute diarrhea in France. J Clin Microbiol. 2002; 40:4266–72.

8. Steffen R, Hill DR, DuPont HL. Traveler's diarrhea: a clinical review. JAMA. 2015; 313:71–80.
9. Cho MC, Noh SA, Kim MN, Kim KM. Direct application of multiplex PCR on stool specimens for detection of enteropathogenic bacteria. Korean J Clin Microbiol. 2010; 13:162–8.

10. Liu J, Gratz J, Maro A, Kumburu H, Kibiki G, Taniuchi M, et al. Simultaneous detection of six diarrhea-causing bacterial pathogens with an in-house PCR-luminex assay. J Clin Microbiol. 2012; 50:98–103.

11. Hori H, Akpedonu P, Armah G, Aryeetey M, Yartey J, Kamiya H, et al. Enteric pathogens in severe forms of acute gastroenteritis in Ghanaian children. Acta Paediatr Jpn. 1996; 38:672–6.

12. Unicomb LE, Faruque SM, Malek MA, Faruque AS, Albert MJ. Demonstration of a lack of synergistic effect of rotavirus with other diarrheal pathogens on severity of diarrhea in children. J Clin Microbiol. 1996; 34:1340–2.

13. Hwang PJ, Kwak JH, Lee TJ, Jeong SJ. Clinical features of acute noroviral gastroenteritis in children: comparison with rotaviral gastroenteritis. Korean J Pediatr. 2009; 52:453–7.
14. Kang JM, Kim BN, Choi SH, Kim NJ, Woo JH, Ryu J, et al. Clinical features and prognostic factors of Aeromonas bacteremia. Infect Chemother. 2005; 37:161–6.
15. Thornley JP, Shaw JG, Gryllos IA, Eley A. Virulence properties of clinically significant Aeromonas species: evidence for pathogenicity. Rev Med Microbiol. 1997; 8:61–72.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download