Abstract
Purpose
Oral allergy syndrome (OAS) is a unique allergic reaction to fresh fruits or vegetables, which is caused by cross-reactivity between foods and pollens. This study was conducted to investigate the clinical feature of OAS and relevant pollen allergens as well as the association between them in Korean children.
Methods
This single-center study included 290 children who were sensitized to pollens at Severance Hospital, and the clinical characteristics of children with and without OAS were compared. A multicenter study included 97 children who were diagnosed with OAS at 3 hospitals between January 2008 and June 2014. The details of clinical features were collected by retrospective medical record reviews using a standardized case report form. The relevant pollen allergens were identified by skin prick tests and/or serum specific IgE levels.
Results
The most commonly sensitized allergen was Japanese hop in pollen-sensitized children. Children with OAS were most commonly sensitized to birch and oak, and 12.4% of the pollen-sensitized children had OAS in the single center. The number of children who were newly diagnosed with OAS has increased over the past 7 years. The most common causative food of OAS was apple. More than 60% of patients with OAS had oral allergic reactions to multiple foods.
Go to : 
REFERENCES
1. Mari A, Ballmer-Weber BK, Vieths S. The oral allergy syndrome: improved diagnostic and treatment methods. Curr Opin Allergy Clin Immunol. 2005; 5:267–73.

2. Yagami T, Haishima Y, Nakamura A, Osuna H, Ikezawa Z. Digestibility of allergens extracted from natural rubber latex and vegetable foods. J Allergy Clin Immunol. 2000; 106:752–62.

3. Ma S, Sicherer SH, Nowak-Wegrzyn A. A survey on the management of pollen-food allergy syndrome in allergy practices. J Allergy Clin Immunol. 2003; 112:784–8.

4. Katelaris CH. Food allergy and oral allergy or pollen-food syndrome. Curr Opin Allergy Clin Immunol. 2010; 10:246–51.

5. Hoffmann-Sommergruber K, O'Riordain G, Ahorn H, Ebner C, Laimer Da Camara Machado M, Pühringer H, et al. Molecular characterization of Dau c 1, the Bet v 1 homologous protein from carrot and its cross-re-activity with Bet v 1 and Api g 1. Clin Exp Allergy. 1999; 29:840–7.
6. Brown CE, Katelaris CH. The prevalence of the oral allergy syndrome and pollen-food syndrome in an atopic paediatric population in south-west Sydney. J Paediatr Child Health. 2014; 50:795–800.

7. Bedolla-Barajas M, Kestler-Gramajo A, Alcalá-Padilla G, Morales-Rome-ro J. Prevalence of oral allergy syndrome in children with allergic diseases. Allergol Immunopathol (Madr). 2017; 45:127–33.

8. Cho YS, Lim YJ, Lee JC, Kim SH, Lim MK, Yoo B, et al. Oral allergy syndrome in pollen - sensitized patients. J Asthma Allergy Clin Immunol. 1998; 18:458–65.
9. Dreborg S. Food allergy in pollen-sensitive patients. Ann Allergy. 1988; 61(6 Pt 2):41–6.
10. Park HJ, Lee JH, Park KH, Kim KR, Han MJ, Choe H, et al. A six-year study on the changes in airborne pollen counts and skin positivity rates in Korea: 2008-2013. Yonsei Med J. 2016; 57:714–20.

11. Kim JH, Oh JW, Lee HB, Kim SW, Kang IJ, Kook MH, et al. Changes in sensitization rate to weed allergens in children with increased weeds pollen counts in Seoul metropolitan area. J Korean Med Sci. 2012; 27:350–5.

12. Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010; 104:101–8.

13. Yun YY, Ko SH, Park JW, Hong CS. IgE immune response to Ginkgo bi-loba pollen. Ann Allergy Asthma Immunol. 2000; 85:298–302.

14. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63(Suppl 86):8–160.
15. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000; 161:309–29.
16. Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups. Is eczema really on the increase world-wide? J Allergy Clin Immunol. 2008; 121:947–54.e15.

17. Choi MH, Kwon EM, Kim HB, Kim CK. Sensitization to inhalant allergens and its association with allergic diseases in preschool children. Korean J Asthma Allergy Clin Immunol. 2012; 32:176–82.
18. Lee JW, Choi GS, Kim JE, Jin HJ, Kim JH, Ye YM, et al. Changes in sensitization rates to pollen allergens in allergic patients in the southern part of Gyeonggi province over the last 10 years. Korean J Asthma Allergy Clin Immunol. 2011; 31:33–40.
19. Jin HJ, Choi GS, Shin YS, Kim JH, Kim JE, Ye YM, et al. The allergenic potency of Japanese hop pollen is increasing with environmental changes in Korea. Allergy Asthma Immunol Res. 2013; 5:309–14.

20. Caliskaner Z, Naiboglu B, Kutlu A, Kartal O, Ozturk S, Onem Y, et al. Risk factors for oral allergy syndrome in patients with seasonal allergic rhinitis. Med Oral Patol Oral Cir Bucal. 2011; 16:e312–6.

21. Ludman S, Jafari-Mamaghani M, Ebling R, Fox AT, Lack G, Du Toit G. Pollen food syndrome amongst children with seasonal allergic rhinitis attending allergy clinic. Pediatr Allergy Immunol. 2016; 27:134–40.

22. American College of Allergy, Asthma, & Immunology. Food allergy: a practice parameter. Ann Allergy Asthma Immunol. 2006; 96(3 Suppl 2):S1–68.
24. Mogensen JE, Wimmer R, Larsen JN, Spangfort MD, Otzen DE. The major birch allergen, Bet v 1, shows affinity for a broad spectrum of physiological ligands. J Biol Chem. 2002; 277:23684–92.

25. Asero R, Antonicelli L, Arena A, Bommarito L, Caruso B, Crivellaro M, et al. EpidemAAITO: features of food allergy in Italian adults attending allergy clinics: a multi-centre study. Clin Exp Allergy. 2009; 39:547–55.

26. Kondo Y, Tokuda R, Urisu A, Matsuda T. Assessment of cross-reactivity between Japanese cedar (Cryptomeria japonica) pollen and tomato fruit extracts by RAST inhibition and immunoblot inhibition. Clin Exp Allergy. 2002; 32:590–4.

27. Maeda N, Inomata N, Morita A, Kirino M, Ikezawa Z. Correlation of oral allergy syndrome due to plant-derived foods with pollen sensitization in Japan. Ann Allergy Asthma Immunol. 2010; 104:205–10.

28. Cuesta-Herranz J, Lázaro M, Figueredo E, Igea JM, Umpiérrez A, De-Las- Heras M. Allergy to plant-derived fresh foods in a birch- and ragweed-free area. Clin Exp Allergy. 2000; 30:1411–6.

29. Egger M, Mutschlechner S, Wopfner N, Gadermaier G, Briza P, Ferreira F. Pollen-food syndromes associated with weed pollinosis: an update from the molecular point of view. Allergy. 2006; 61:461–76.

30. Hong GN, Kim MA, Yoon MK, Lee SH, Park HS. Oral allergy syndrome caused by crown daisy and sesame leaf. Allergy Asthma Respir Dis. 2014; 2:306–9.

31. Lee KH, Lee YW, Hong CS, Park JW. A case of successful immunotherapy in the oral allergy syndrome. Korean J Asthma Allergy Clin Immunol. 2005; 25:150–2.
Go to : 
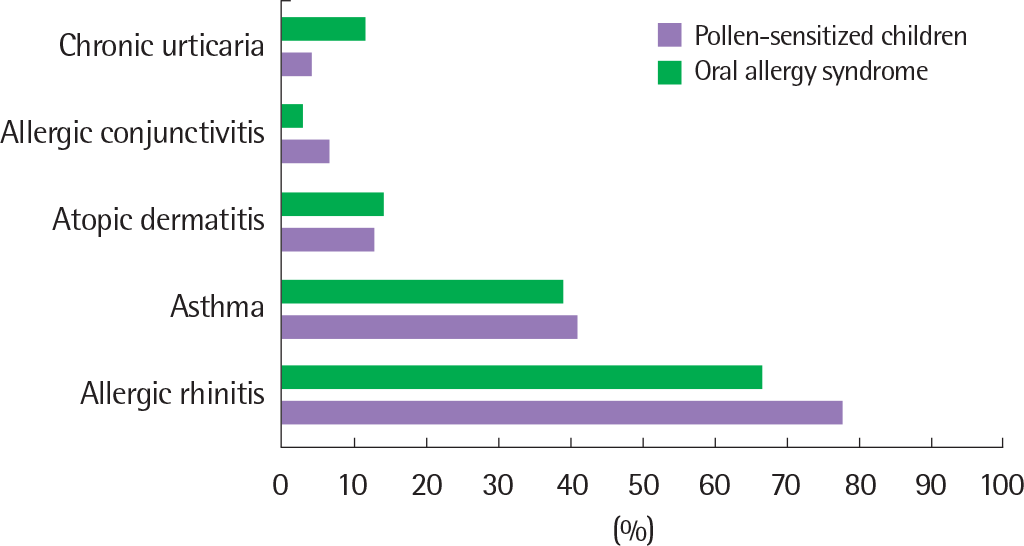 | Fig. 1.Distribution of allergic diseases in pollen-sensitized children (n=290) and distribution of combined other allergic diseases in children with oral allergy syndrome (n=36) in a single center. |
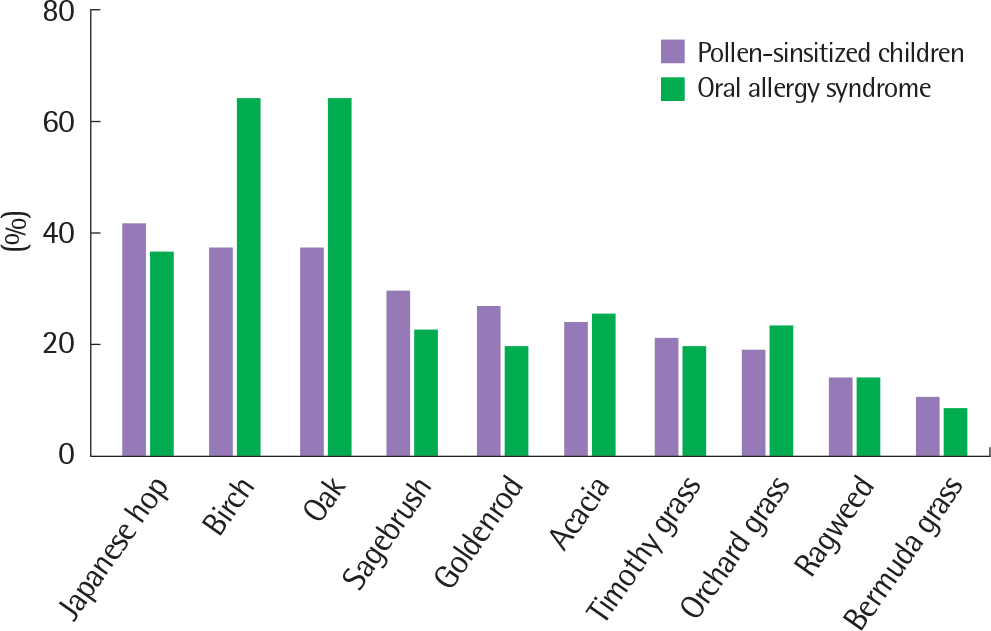 | Fig. 2.Sensitization rates to pollen allergens. The most common sensitized allergens were Japanese hop followed by birch, oak, sagebrush in pollen-sensi-tized children (n=290). Birch and oak were the most commonly sensitized allergens in children with oral allergy syndrome (n=36) in a single center. |
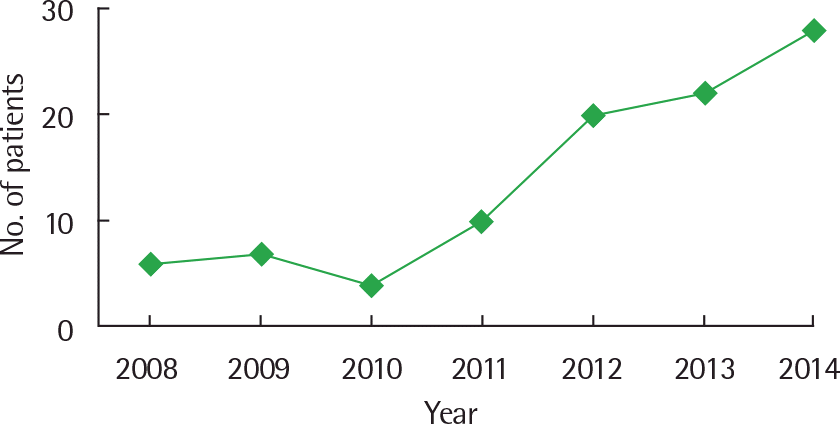 | Fig. 3.Yearly incidence of oral allergy syndrome showing an increasing tendency (n=97, multicenter study). |
Table 1.
Comparison of children with or without oral allergy syndrome (OAS) in a single center (n=290)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download