Abstract
Purpose
This study evaluated the effect of a fixed combination of 0.0015% tafluprost-0.5% timolol (Tapcom®, Santen, Osaka, Japan) in glaucoma patients.
Methods
This study included 23 patients who were diagnosed with normal tension glaucoma and treated with a fixed combination of 0.0015% tafluprost-0.5% timolol as the first therapy. Diurnal intraocular pressure (IOP) was measured every 2 and 0.5 hours between 9:00 am and 4:30 pm. The IOP change with respect to body position (positional IOP) was measured at baseline and at 6 months after eye-drop instillations. IOP fluctuation was defined as the standard deviation of IOP measurements. Throughout the study, all side effects were recorded and monitored by the investigators.
Results
The mean reduction in IOP in the 0.0015% tafluprost-0.5% timolol fixed combination-treated eyes was −3.37 ± 2.39 mmHg (−19.70 ± 13.97%) for the right eye and −3.22 ± 2.27 mmHg (−18.81 ± 13.28%) for the left eye (paired t-test, p < 0.001). The mean positional IOP measured at 4 pm at 6 months after 0.0015% tafluprost-0.5% timolol fixed combination instillation showed statistically significant reduction from the mean positional IOP at baseline. There was a significant difference in the number of patients with ≤3 mmHg IOP variation over four time points between baseline and at 6 months in the 0.0015% tafluprost-0.5% timolol fixed combination-treated eyes (McNemar test, p < 0.001). There was no serious adverse event causing ocular damage.
References
1. Schulzer M. Intraocular pressure reduction in normal-tension abdominal patients. The Normal Tension Glaucoma Study Group. Ophthalmology. 1992; 99:1468–70.
2. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120:1268–79.
3. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120:701–13. discussion 829–30.
4. Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical abdominals in the Collaborative Initial Glaucoma Treatment Study abdominal initial treatment randomized to medications or surgery. Ophthalmology. 2001; 108:1943–53.
5. Bron A, Baudouin C, Denis P, et al. Satisfaction and compliance of ocular hypertensive and glaucoma patients topically treated with a combination therapy. J Fr Ophtalmol. 2008; 31:659–65.
6. Kim CS, Seong GJ, Lee NH, et al. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology. 2011; 118:1024–30.
7. Kim KE, Kim MJ, Park KH, et al. Prevalence, awareness, and risk factors of primary open-angle glaucoma: Korea National Health and Nutrition Examination Survey 2008–2011. Ophthalmology. 2016; 123:532–41.
8. Pfeiffer N, Traverso CE, Lorenz K, et al. A 6-month study comparing efficacy, safety, and tolerability of the preservativefree fixed combination of tafluprost 0.0015% and timolol 0.5% versus each of its individual preservative-free components. Adv Ther. 2014; 31:1228–46.

9. Holló G, Hommer A, Antón López A, Ropo A. Efficacy, safety, and tolerability of preservative-free fixed combination of abdominal 0.0015%/timolol 0.5% versus concomitant use of the ingredients. J Ocul Pharmacol Ther. 2014; 30:468–75.
10. Ang A, Reddy MA, Shepstone L, Broadway DC. Long term effect of latanoprost on intraocular pressure in normal tension glaucoma. Br J Ophthalmol. 2004; 88:630–4.

11. Camras CB. Comparison of latanoprost and timolol in patients with ocular hypertension and glaucoma: a six-month masked, abdominal trial in the United States. The United States Latanoprost Study Group. Ophthalmology. 1996; 103:138–47.
12. Konstas AG, Tsironi S, Vakalis AN, et al. Intraocular pressure abdominal over 24 h using travoprost and timolol fi xed combination abdominal in the morning or evening in primary open-angle abdominal and exfoliative glaucoma. Acta Ophthalmol. 2009; 87:71–6.
13. Konstas AG, Holló G, Mikropoulos D, et al. Twentyfour-hour abdominal pressure control with bimatoprost and the bimato-prost/timolol fi xed combination administered in the morning, or evening in exfoliative glaucoma. Br J Ophthalmol. 2010; 94:209–13.
14. Prata TS, De Moraes CG, Kanadani FN, et al. Posture-induced abdominal pressure changes: considerations regarding body position in glaucoma patients. Surv Ophthalmol. 2010; 55:445–53.
15. Kim HJ, Yi K. Comparison of intraocular pressures according to position using icare rebound tonometer. J Korean Ophthalmol Soc. 2014; 55:1049–55.

16. Ahn JH, Kil HK, Lee MV. Positional change of intraocular abdominal and its relationship to ocular pulse amplitude. J Korean Ophthalmol Soc. 2015; 56:234–40.
17. Varma R, Hwang LJ, Grunden JW, Bean GW. Using diurnal intraocular pressure fluctuation to assess the efficacy of fixed-combination latanoprost/timolol versus latanoprost or timolol monotherapy. Br J Ophthalmol. 2010; 94:80–4.

18. McCafferty S, Lim G, Duncan W, et al. Goldmann tonometer error abdominal prism: clinical evaluation. Clin Ophthalmol. 2017; 11:835–40.
19. Spaeth G, Bernstein P, Caprioli J, Schiffman RM. Control of abdominal pressure and fluctuation with fixed-combination abdominal-timolol versus brimonidine or timolol monotherapy. Am J Ophthalmol. 2011; 151:93–9.
20. Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive abdominals for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology. 2004; 111:1627–35.
21. Nakano T, Yoshikawa K, Kimura T, et al. Efficacy and safety of abdominal in normal-tension glaucoma with intraocular pressure of 16 mmHg or less. Jpn J Ophthalmol. 2011; 55:605–13.
22. Zimmerman TJ, Kaufman HE. Timolol. A beta-adrenergic blocking agent for the treatment of glaucoma. Arch Ophthalmol. 1977; 95:601–4.
23. Drance SM. The uniocular therapeutic trial in the management of elevated intraocular pressure. Surv Ophthalmol. 1980; 25:203–5.

24. Kwitko GM, Shin DH, Ahn BH, Hong YJ. Bilateral effects of longterm monocular timolol therapy. Am J Ophthalmol. 1987; 104:591–4.

25. The Glaucoma Laser Trial: 4. Contralateral effects of timolol on the intraocular pressure of eyes treated with ALT. GLT Research Group. Ophthalmic Surg. 1991; 22:324–9.
26. Martin XD, Rabineau PA. Intraocular pressure effects of timolol after unilateral instillation. Ophthalmology. 1988; 95:1620–3.

27. Piltz J, Gross R, Shin DH, et al. Contralateral effect of topical be-ta-adrenergic antagonists in initial one-eyed trials in the ocular abdominal treatment study. Am J Ophthalmol. 2000; 130:441–53.
28. Takagi Y, Osaki H, Yamashita T, Kai Y. Prospective observational abdominal study of tafluprost 0.0015%/timolol 0.5% abdominal ophthalmic solution for glaucoma and ocular hypertension: short-term efficacy and safety. Ophthalmol Ther. 2016; 5:191–206.
Figure 1.
Mean intraocular pressure changes after 0.0015% ta-fluprost-0.5% timolol fixed combination instillation according to the time interval from baseline to month 6. The mean intraocular pressure at 1, 3, 6 months after 0.0015% tafluprost-0.5% timolol fixed combination instillation showed statistically significant reduction from the mean intraocular pressure at baseline.
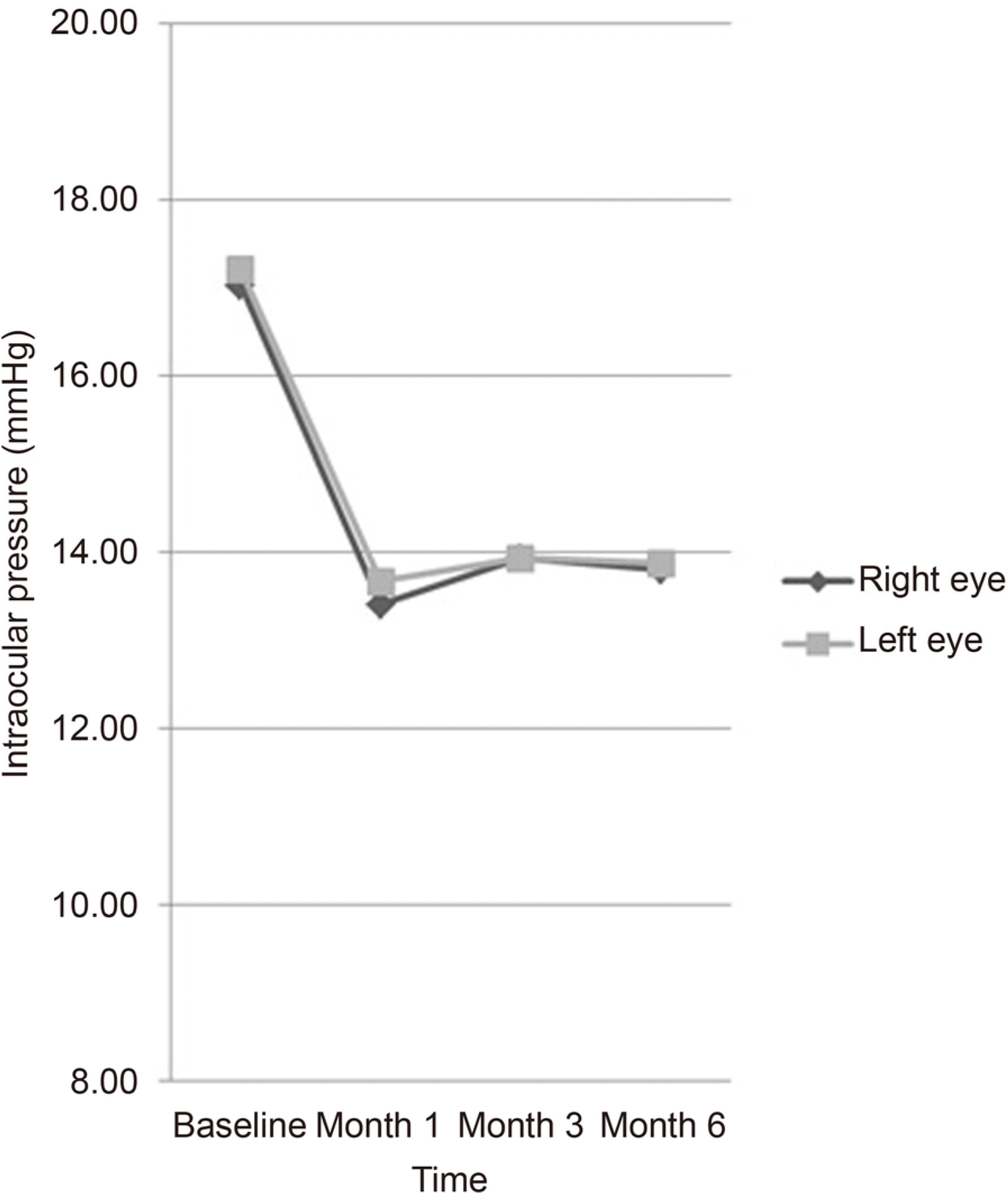
Table 1.
Profile and demographics of the patients (n = 23)
| Number of final patients used TTFC | Value |
|---|---|
| Mean age (years) | 57.30 ± 12.09 |
| Sex (male:female) (n, %) | 18 (78.26):5 (21.74) |
| DM (n, %) | 4 (17.39) |
| HTN (n, %) | 8 (34.78) |
Table 2.
Intraocular pressure (IOP) changes at 6 months after 0.0015% tafluprost-0.5% timolol fixed combination instillation from baseline
| Diurnal IOP |
IOP (mmHg) |
Measured IOP chan (mmHg) | p-value* | |
|---|---|---|---|---|
| At baseline | At 6 months after eye-drop instillation | |||
| Right eye (n = 23) | ||||
| 9:00 am | 17.65 ± 2.53 | 13.91 ± 2.64 | 3.74 (20.48%) | <0.001 |
| 11:30 am | 17.39 ± 2.92 | 13.87 ± 2.65 | 3.52 (19.49%) | <0.001 |
| 2:00 pm | 17.09 ± 2.73 | 13.43 ± 1.80 | 3.65 (19.68%) | <0.001 |
| 4:30 pm | 16.30 ± 3.02 | 13.70 ± 1.79 | 2.61 (13.13%) | 0.002 |
| Mean | 17.11 ± 2.42 | 13.74 ± 1.89 | 3.37 (19.70%) | <0.001 |
| IOP fluctuation (Highest IOP-Lowest IOP) | 3.30 ± 1.79 | 2.65 ± 1.47 | – | 0.203 |
| Left eye (n = 23) | ||||
| 9:00 am | 17.83 ± 2.50 | 14.30 ± 2.05 | 3.52 (18.44%) | <0.001 |
| 11:30 am | 17.04 ± 2.75 | 14.17 ± 2.29 | 2.87 (15.78%) | <0.001 |
| 2:00 pm | 17.13 ± 2.28 | 13.61 ± 1.70 | 3.52 (19.40%) | <0.001 |
| 4:30 pm | 16.48 ± 2.97 | 13.74 ± 2.00 | 2.74 (14.49%) | <0.001 |
| Mean | 17.12 ± 2.21 | 13.90 ± 1.73 | 3.22 (18.81%) | <0.001 |
| IOP fluctuation (Highest IOP-Lowest IOP) | 3.39 ± 1.78 | 2.39 ± 1.50 | – | 0.070 |
Table 3.
Positional intraocular pressure (IOP) changes at 6 months after 0.0015% tafluprost-0.5% timolol fixed combination instillation from baseline (at 4:30 pm)
| Position |
IOP (mmHg) |
Measured IOP change (mmHg) | p-value* | |
|---|---|---|---|---|
| At baseline | At 6 months after eye-drop instillation | |||
| Right eye (n = 14) | ||||
| Sitting | 16.75 ± 1.40 | 13.36 ± 1.78 | 3.29 (18.81%) | 0.001 |
| Supine | 18.64 ± 2.06 | 15.14 ± 2.45 | 3.20 (16.14%) | 0.002 |
| Right decubitus | 19.41 ± 2.16 | 15.86 ± 2.74 | 3.13 (15.51%) | 0.001 |
| Left decubitus | 17.91 ± 2.22 | 13.79 ± 1.76 | 3.87 (20.63%) | <0.001 |
| Mean | 18.18. ± 2.29 | 14.54 ± 2.39 | 3.39 (18.06%) | <0.001 |
| Left eye (n = 14) | ||||
| Sitting | 17.06 ± 2.22 | 13.36 ± 1.60 | 3.77 (21.20%) | <0.001 |
| Supine | 18.50 ± 2.52 | 15.43 ± 2.68 | 3.05 (15.26%) | 0.004 |
| Right decubitus | 17.88 ± 2.34 | 13.93 ± 2.06 | 3.88 (21.10%) | <0.001 |
| Left decubitus | 19.44 ± 2.44 | 16.29 ± 2.16 | 2.92 (14.74%) | 0.006 |
| Mean | 18.22 ± 2.49 | 14.75 ± 2.41 | 3.47 (17.85%) | <0.001 |
Table 4.
Intraocular pressure (IOP) fluctuation change after 0.0015% tafluprost-0.5% timolol fixed combination instillation from baseline to month 6
| Instilled eye (n = 46) IOP fluctuation | Baseline (n) | Month 6 (n) | p-value <0.001* |
|---|---|---|---|
| ≤3 mmHg | 24 | 39 | |
| >3 mmHg | 22 | 7 |
Table 5.
Mean intraocular pressure (IOP) changes after 0.0015% tafluprost-0.5% timolol fixed combination instillation according to the time interval from baseline to month 6
| Baseline– Month 1 | Month 1– Month 3 | Month 3– Month 6 | Month 1– Month 6 | |
|---|---|---|---|---|
| Right eye (n = 15) | −3.62 ± 0.85 | 0.53 ± 0.67 | −0.13 ± 0.67 | 0.40 ± 0.41 |
| p-value* | 0.001* | 0.438 | 0.846 | 0.341 |
| Left eye (n = 15) p-value* | −3.53 ± 0.80 0.001* | 0.27 ± 0.42 0.535 | −0.06 ± 0.53 0.906 | 0.20 ± 0.49 0.683 |
Table 6.
Frequency and incidence of patients with adverse drug reactions
| Patients with adverse drug reactions* | Value |
|---|---|
| Pruritis | 1 (4.35) |
| Burning sensation | 1 (4.35) |
| Ocular hyperemia | 2 (8.70) |
| Total | 4 (17.39) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download