Abstract
Purpose
To evaluate the efficacy of intravitreal preservative-free triamcinolone acetonide (Maqaid®) injection for the treatment of macular edema secondary to retinal vein occlusion (RVO) refractory to intravitreal bevacizumab injections.
Methods
This retrospective, observational study included 17 eyes of 17 patients with refractory macular edema secondary to RVO. The patients with macular edema unresponsive to intravitreal bevacizumab injections were treated with intravitreal preser-vative-free triamcinolone acetonide (Maqaid®) injection. Best-corrected visual acuity (BCVA) and central foveal thickness (CFT) based on optical coherence tomography were evaluated before intravitreal triamcinolone injection (IVTA), 1 month and 3 months after IVTA injection. intraocular pressure (IOP) changes were analyzed up to 6 months.
Results
The logarithm of the minimal angle of resolution (logMAR) BCVA was decreased from 0.56 ± 0.32 to 0.41 ± 0.32 after 1 month with statistical significance (p = 0.006) and to 0.47 ± 0.36 after 3 months of IVTA without statistical significance (p = 0.204). CFT was significantly improved from 474.82 ± 91.91 μ m to 262.58 ± 60.11 μ m after 1 month and to 339.58 ± 152.48 μ m after 3 months of IVTA injection (p ≤ 0.001 and 0.005, respectively). IOP was significantly increased from 13.11 ± 2.66 mmHg to 16.64 ± 5.66 mmHg after 1 month and to 17.05 ± 7.21 μ m after 3 months of IVTA injection (p = 0.024 and 0.026, respectively). Treatment-associated IOP elevation was manageable with antiglaucoma medications. IOP was 15.13 ± 3.90 mmHg after 6 months of IVTA injection (p = 0.023).
Conclusions
Intravitreal preservative-free triamcinolone (Maqaid®) Injection improves BCVA and reduces CFT in some patients with macular edema secondary to RVO refractory to intravitreal bevacizumab therapy. There were no serious vision-threatening complications associated with intravitreal preservative-free triamcinolone (Maqaid®) therapy during the study period. Intravitreal preservative-free triamcinolone (Maqaid®) could be considered as a treatment option for refractory macular edema associated with RVO.
Go to : 
References
1. Mitchell P, Smith W, Chang A. Prevalence and associations of abdominall vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol. 1996; 114:1243–7.
2. Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008; 33:111–31.

3. Noma H, Funatsu H, Yamasaki M, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005; 104:256–61.

4. Yoshimura T, Sonoda KH, Sugahara M, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009; 4:e8158.

5. Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. Am J Ophthalmol. 1984; 98:271–82.
6. Evaluation of grid pattern photocoagulation for macular edema in central vein occlusion. The Central Vein Occlusion Study Group M report. Ophthalmology. 1995; 102:1425–33.
7. Guthoff R, Meigen T, Hennemann K, Schrader W. Comparison of bevacizumab and triamcinolone for treatment of macular edema secondary to branch retinal vein occlusion in a pair-matched analysis. Ophthalmologica. 2010; 224:319–24.

8. Ding X, Li J, Hu X, et al. Prospective study of intravitreal abdominal acetonide versus bevacizumab for macular edema abdominal to central retinal vein occlusion. Retina. 2011; 31:838–45.
9. Demir M, Dirim B, Acar Z, et al. Comparison of the effects of abdominal bevacizumab and triamcinolone acetonide in the abdominal of macular edema secondary to central retinal vein occlusion. Indian J Ophthalmol. 2014; 62:279–83.
10. Campa C, Alivernini G, Bolletta E, et al. Anti-VEGF therapy for retinal vein occlusions. Curr Drug Targets. 2016; 17:328–36.

11. Lim HB, Kim MS, Jo YJ, Kim JY. Prediction of retinal ischemia in branch retinal vein occlusion: spectraldomain optical coherence tomography study. Invest Ophthalmol Vis Sci. 2015; 56:6622–9.

12. Hanada N, Iijima H, Sakurada Y, Imasawa M. Recurrence of abdominal edema associated with branch retinal vein occlusion after abdominal bevacizumab. Jpn J Ophthalmol. 2012; 56:165–74.
13. Kondo M, Kondo N, Ito Y, et al. Intravitreal injection of abdominal for macular edema secondary to branch retinal vein abdominal:results after 12 months and multiple regression analysis. Retina. 2009; 29:1242–8.
14. Matsumoto Y, Freund KB, Peiretti E, et al. Rebound macular abdominal following bevacizumab (Avastin) therapy for retinal venous occlusive disease. Retina. 2007; 27:426–31.
15. Campochiaro PA, Sophie R, Pearlman J, et al. abdominal abdominals in patients with retinal vein occlusion treated with abdominal: the RETAIN study. Ophthalmology. 2014; 121:209–19.
16. Kang TK, Kim YC, Kim KS. Factors related to repeatability of abdominal bevacizumab injections in branch retinal vein occlusion macular edema. J Korean Ophthalmol Soc. 2015; 56:1580–5.
17. Moon BG, Cho AR, Kim YN, Kim JG. Predictors of refractory macular edema after branch retinal vein occlusion following abdominal bevacizumab. Retina. 2018; 38:1166–74.
18. Zhu MD, Cai FY. Development of experimental chronic intraocular hypertension in the rabbit. Aust N Z J Ophthalmol. 1992; 20:225–34.

19. Otsuka H, Kawano H, Sonoda S, et al. Particle-induced endophthalmitis: possible mechanisms of sterile endophthalmitis after abdominal triamcinolone. Invest Ophthalmol Vis Sci. 2013; 54:1758–66.
20. Maia M, Farah ME, Belfort RN, et al. Effects of intravitreal abdominal acetonide injection with and without preservative. Br J Ophthalmol. 2007; 91:1122–4.
21. Chang YS, Wu CL, Tseng SH, et al. In vitro benzyl alcohol abdominal: implications for intravitreal use of triamcinolone acetonide. Exp Eye Res. 2008; 86:942–50.
22. Shimamura S, Kawai K, Odontuya D, Ichihashi T. A comparison of characteristic properties and qualitative difference between three kinds of triamcinolone acetonide. Tokai J Exp Clin Med. 2017; 42:67–70.
23. Noma H, Funatsu H, Yamasaki M, et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye (Lond). 2008; 22:42–8.

24. Fonollosa A, Garcia-Arumi J, Santos E, et al. Vitreous levels of inter-leukine-8 and monocyte chemoattractant protein-1 in macular oabdominal with branch retinal vein occlusion. Eye (Lond). 2010; 24:1284–90.
25. Lee H, Shah GK. Intravitreal triamcinolone as primary treatment of cystoid macular edema secondary to branch retinal vein occlusion. Retina. 2005; 25:551–5.

26. Yepremyan M, Wertz FD, Tivnan T, et al. Early treatment of abdominal macular edema secondary to branch retinal vein occlusion with triamcinolone acetonide. Ophthalmic Surg Lasers Imaging. 2005; 36:30–6.
27. Jonas JB, Akkoyun I, Kamppeter B, et al. Branch retinal vein abdominal treated by intravitreal triamcinolone acetonide. Eye. 2005; 19:65–71.
28. Ahn HM, Choi KS. abdominal effectiveness of intravitreal abdominal injection for refractory macular edema secondary to branch retinal vein occlusion. J Korean Ophthalmol Soc. 2016; 57:1731–7.
29. Wingate RJ, Beaumont PE. Intravitreal triamcinolone and elevated intraocular pressure. Aust N Z J Ophthalmol. 1999; 27:431–2.
30. Aref AA, Scott IU, Oden NL, et al. Incidence, risk factors, and timing of elevated intraocular pressure after intravitreal triamcinolone abdominal injection for macular edema secondary to retinal vein occlusion: SCORE Study Report 15. JAMA Ophthalmol. 2015; 133:1022–9.
31. Shaarawy T, Sherwood M, Hitchings R, Crowston J. Glaucoma. 2nd ed.Vol. 1. Philadelphia: Saunders;2014. p. 438.
Go to : 
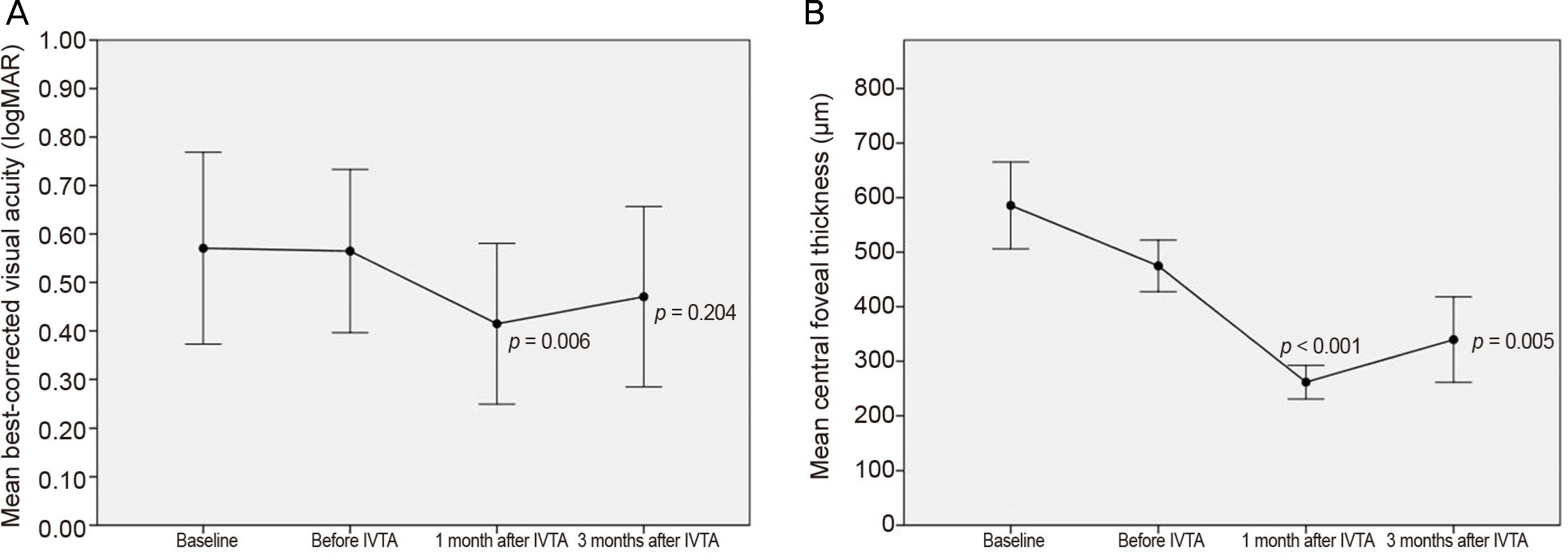 | Figure 1.Changes in logarithm of the minimal angle of resolution best-corrected visual acuity and central foveal thickness in eyes with macular edema secondary to retinal vein occlusion. Measurements were made at diagnosis (baseline), before intravitreal triamcinolone injection (IVTA) which is after intravitreal bevacizumab injections, 1 month after IVTA, and 3 months after IVTA. (A) Although the visual acuity was significantly improved 1 month after IVTA, no statistical significance was found 3 months after IVTA. (B) The central foveal thickness was significantly improved after IVTA (Wilcoxon signed-rank test). |
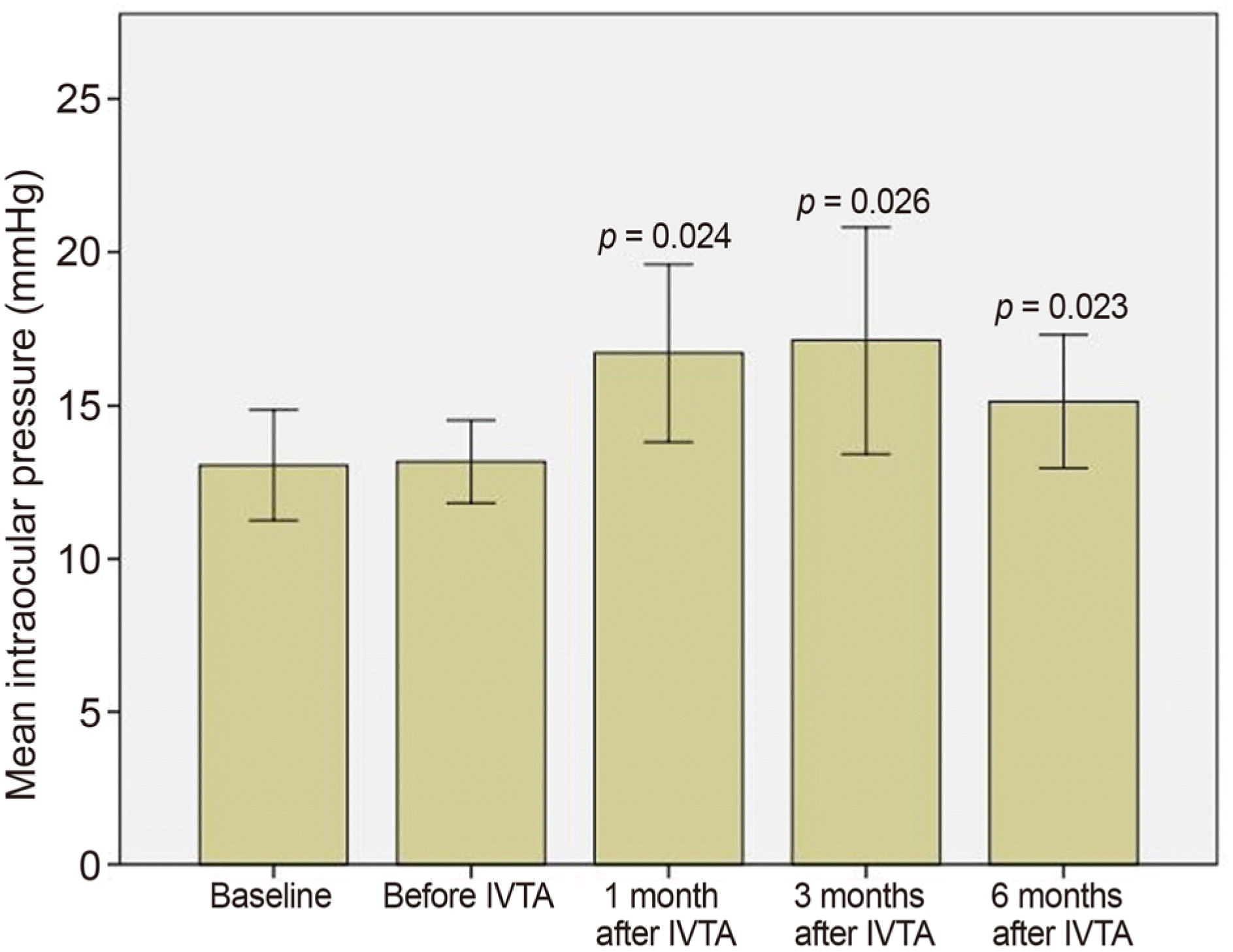 | Figure 2.Changes in intraocular pressure in eyes with macular edema secondary to retinal vein occlusion. Measurements were made at diagnosis (baseline), before intravitreal triamcinolone injection (IVTA) which is after intravitreal bevacizumab injections, 1 month after IVTA, 3 months after IVTA, and 6 months after IVTA. The intraocular pressure was significantly increased after IVTA (Wilcoxon signed-rank test). |
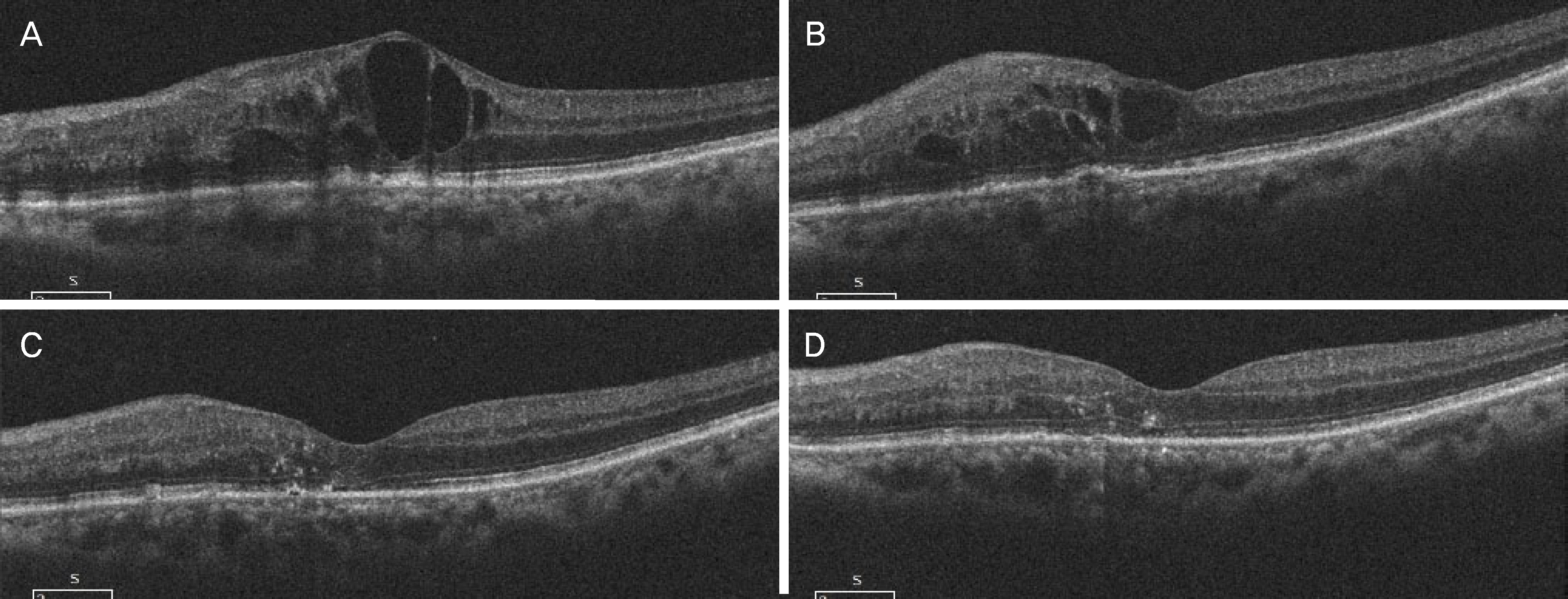 | Figure 3.Optical coherence tomography (OCT) images of an eye diagnosed with refractory macular edema secondary to central retinal vein occlusion. (A) Compared to baseline. (B) Macular edema remained after 2 consecutive intravitreal bevacizumab injections. (C) A marked decrease in macular edema was noted 1 month after an intravitreal injection of triamcinolone. (D) OCT showed a flat macula without macular edema 3 month after an intravitreal injection of triamcinolone. |
Table 1.
Baseline characteristics of patients with macular edema secondary to retinal vein occlusion that was refractory to intravitreal bevacizumab therapy (n = 17 eyes)
| Data | |
|---|---|
| Age (years) | 64.9 ± 11.9 |
| Gender (male:female) | 10:7 |
| Classification | |
| Branch RVO (n, %) | 13 (76.4) |
| Central RVO (n, %) | 4 (23.6) |
| Type | |
| Non-ischemic type | 10 |
| Ischemic type | 7 |
| Baseline BCVA (logMAR) | 0.57 ± 0.38 |
| Mean baseline CFT (μ m)* | 585.64 ± 154.69 |
| Baseline IOP (mmHg) | 12.88 ± 3.68 |
| BCVA (logMAR) before IVTA | 0.56 ± 0.32 |
| Mean CFT (μ m) before IVTA† | 474.82 ± 91.91 |
| IOP before IVTA | 13.11 ± 2.66 |
| Phakic (n, %) | 12 (70.5) |
| Number of bevacizumab injections before IVTA (N) | 1.82 ± 1.07 |
| Time from IVBI to IVTA (week) | 16.58 ± 9.10 |
Table 2.
Changes in logarithm of central foveal thickness and the minimal angle of resolution best-corrected visual acuity and intraocular pressure in eyes with macular edema secondary to branch retinal vein occlusion (n = 13 eyes)
| BCVA (logMAR) | p-value* | CFT (μ m) | p-value* | IOP (mmHg) | p-value* | |
|---|---|---|---|---|---|---|
| Baseline | 0.61 ± 0.38 | 597.07 ± 169.15 | 11.69 ± 2.81 | |||
| Before IVTA | 0.50 ± 0.29 | 474.92 ± 95.20 | 12.69 ± 2.52 | |||
| 1 M after IVTA | 0.37 ± 0.28 | 0.010 | 263.61 ± 66.50 | 0.001 | 16.69 ± 5.83 | 0.039 |
| 3 M after IVTA | 0.42 ± 0.33 | 0.197 | 371.46 ± 160.84 | 0.023 | 15.23 ± 6.15 | 0.178 |
| 6 M after IVTA | 15.00 ± 3.83 | 0.021 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download