Abstract
Purpose
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social communication impairments and repetitive behaviors or restricted interests. Impaired pragmatic language comprehension is a universal feature in individuals with ASD. However, the underlying neural basis of pragmatic language is poorly understood. In the present study, we examined neural activation patterns associated with impaired pragmatic language comprehension in ASD, compared to typically developing children (TDC).
Materials and Methods
Functional magnetic resonance imaging (fMRI) was applied to 15 children with ASD and 18 TDC using the Korean pragmatic language task.
Results
Children with ASD were less accurate than TDC at comprehending idioms, particularly when they were required to interpret idioms with mismatched images (mismatched condition). Children with ASD also showed different patterns of neural activity than TDC in all three conditions (neutral, matched, and mismatched). Specifically, children with ASD showed decreased activation in the right inferior frontal gyrus (IFG) (Brodmann area 47) in the mismatched condition, compared with TDC (IFG; t(31)=3.17, p<0.001).
Autism spectrum disorder (ASD) is a neurodevelopmental disorder defined by qualitative impairment in social communication, as well as restricted and repetitive behaviors and interests.1 Although it has a broad range of severity, impaired language development is a core symptom of ASD.2 Language and communication can be divided into several domains, such as phonology, prosody, syntax, semantics, and pragmatics. Of these, pragmatics is impaired in all individuals with ASD regardless of their level of functioning.34 Pragmatics denotes functional language skills in a social context, and encompasses conventions, rules, and linguistic/non-linguistic means of engaging in socially reciprocal communication.5 Even individuals with high-functioning ASD have impairments in the domains of pragmatics, in contrast to their relatively spared formal linguistic skills.67
For fluently reciprocal communication, the listener is required to understand the speaker's intended meaning that lies behind the literal meaning of the spoken words or sentences. However, individuals with ASD frequently tend to interpret a speaker's utterance overly literally.8 This characteristic of ASD can be explained with reference to theory of mind (ToM), social knowledge, context, facial expressions, vocal prosody, figurative competence, and so on.8 It is known that all of these components are impaired in individuals with ASD.591011 The most recent revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) included some important changes to the diagnostic criteria for ASD.1 One such change is that impairments in social communication and social interaction are now integrated into one criterion in the DSM-5. This change suggests that social interaction and communication are intimately associated and thus difficult to separate.512 Therefore, impaired social reciprocity may be associated with impaired pragmatics in individuals with ASD.
Several functional brain imaging studies have revealed aberrant neural activity during social language tasks in areas associated with sociality and language processing, although results among studies have been inconsistent.41013 As such, no cortical regions have been shown to have a clear relationship with impaired communication in ASD.10 Some researchers have suggested that impaired neural activity underlying perceptual processing, such as of facial and vocal emotional expression, is related to deficits in pragmatics in ASD.1415 Other researchers have deemed that aberrant activity in “social brain areas” is associated with poor pragmatics in ASD. Social brain areas are known to be associated with mentalization/ToM, and individuals with ASD show abnormal brain activity in the medial prefrontal gyrus, one of the social brain areas, compared with control groups.1617 As described so far, functional brain imaging studies reflect associations with impaired pragmatics and social brain areas in ASD. However, there are no functional magnetic resonance imaging (fMRI) studies of Korean populations using pragmatic language tasks, despite the high prevalence of ASD in South Korea.18
In this study, we explored brain activity related to social and language processing, using a social language task. Moreover, we investigated the neural substrates of impaired pragmatic language comprehension in children with ASD, compared to typically developing children (TDC). To determine the neural mechanisms of social and language processing, we conducted an fMRI study with high functioning children with ASD and TDC using the Korean Autism Social Language Task (KASLAT), which is a pragmatic language task that incorporates Korean idioms.12
fMRI data were collected from 17 children with high functioning ASD and 19 TDC. Two participants with ASD and one typically developing child with excess head motion were excluded from the data analyses, resulting in 15 participants with ASD (1 girl, 14 boys; mean age±SD: 9.66±2.19 years) and 18 with TDC (8 girls, 10 boys; mean age±SD: 10.47±2.78 years). All children were recruited from a child and adolescent psychiatric clinic at Severance Children's Hospital, which is affiliated with Yonsei University College of Medicine. For the ASD group, diagnosis was obtained independently by two child and adolescent psychiatrists based on the DSM-5.1 The diagnosis was supplemented by the Autism Diagnostic Interview-Revised and Autism Diagnostic Observation Schedule.1920 The following characteristics were exclusionary for the ASD group: 1) current brain damage or convulsive disorder, or history of same; 2) intellectual disability or language delay; or 3) comorbid child and adolescent psychiatric disorders. All TDC were screened by two child and adolescent psychiatrists with assessments including a psychological assessment and a neurological examination (Table 1). In order to distinguish between TDC and children with ASD clearly, we applied a social responsiveness scale to TDC and excluded children with a score of 40 or more: the scale has proven feasibility for quantitative ascertainment of autistic social impairment in public health settings.21 None of the TDC had past or current developmental, medical, or psychiatric diagnoses. All participants were right-handed native speakers of Korean and had normal or corrected-to-normal vision. The children in the ASD and TDC groups were not significantly different in age and full-scale intelligence quotient (IQ). Participant characteristics, including age, sex, and full-scale IQ, are presented in Table 1. Participants were not sedated for the MRI scanning, and none were taking any psychoactive medications on the day of the scanning. This study was approved by the applicable institutional review boards for research with human subjects at Severance Hospital, Yonsei University College of Medicine (4-2012-0828), where this study was performed. All subjects and their parents were given a full description of the study and provided prior written informed assent and consent in accordance with the Declaration of Helsinki, respectively.
We used the KASLAT as the stimulus in this study. The KASLAT was developed by our research team for assessing pragmatic abilities using idiom comprehension; the tool's reliability and utility was confirmed in a previous study.12 As shown in Fig. 1, the KASLAT is comprised of three conditions: neutral (N), matched (M), and mismatched (MM). The neutral task consists of sentences with a dictionary definition and matching pictures. Therefore, there is no conflict between the literal and figurative meanings of the sentence. The matched task consists of an idiom and a congruent image. In contrast, the mismatched task consists of idioms and mismatched images. Mismatched images depict the literal rather than the metaphorical meaning of the sentence. A block design was used for the sentence-judgment task while acquiring fMRI data. The fMRI scanning procedure was carried out as follows: Before the scanning, participants were told that a picture and sentence would be shown together. They were instructed to press “button 1” on response pads if the sentence and image were matched and to press “button 2” on response pads if the sentence and following image were mismatched. The stimuli within each condition were presented randomly. The sentence and image were presented for 2 seconds and each condition consisted of three trials. All conditions were repeated five times during the task; the total task-duration was 468 seconds.
We collected imaging data using a 3-tesla MRI scanner (Philips Healthcare, Best, the Netherlands). Functional and anatomical images were acquired using different MRI sequences. Functional images were obtained using a gradient echo single-shot echo planar imaging sequence [repetition time (TR)=2000 ms, echo time (TE)=30 ms, in-plane resolution=3×3 mm2, slice thickness=3 mm, field of view (FOV)=240 mm, 32 slices]. After acquiring functional images, we also obtained a T1-weighted anatomical image with specific scan parameters (TR=9.7 ms, TE=4.6 ms, in-plane resolution=0.86×0.86 mm2, slice thickness=1 mm, FOV=220 mm, 220 slices). T1-weighted and functional images had the same orientation for better coregistration in 3D-space.
Functional data were analyzed using Brain Voyager QX (Brain Innovation B.V., Maastricht, the Netherlands). Anatomical data were spatially transformed to iso-voxel space with cubic spline interpolation and normalized by Talairach transformation. Functional data were preprocessed by slice-timing correction, head motion correction, and temporal high-pass filtering (fast Fourier transform, cut-off=0.01 Hz). These functional data were coregistered with each normalized anatomical image and spatially smoothed using a Gaussian kernel with 6 mm full-width at half-maximum. For individual analyses, activation maps of subjects were constructed using a general linear model that contained the contrasts of our study design. In group analyses, all individual maps were analyzed in Brain Voyager QX using a two-way ANOVA with a random effect. The group×condition interaction, the main effect of the group, and the overall main effect were estimated using whole-brain analysis. In addition, simple effects analysis was conducted to determine group differences in every condition. All activation maps were corrected for multiple comparisons at a cluster-level threshold of p<0.005.
Reaction time (RT) and accuracy data are shown in Fig. 2. To examine differences in RTs and accuracy, two-way ANOVAs were conducted. RTs showed significant main effects for group and condition [F(1, 99)=7.55, p<0.01; F(2, 99)=5.22, p<0.01, respectively] but a non-significant interaction between group and condition [F(2, 99)=2.48, p>0.05; data not shown]. A two-way ANOVA of accuracy revealed only a significant main effect of group [F(1, 99)=13.72, p<0.001]; the interaction between group and condition was non-significant [F(2, 99)=0.34, p>0.05; data not shown].
To assess group differences in each condition, post-hoc t-tests were conducted for both RT and accuracy. In the N condition, TDC showed significantly slower RTs than participants with ASD [t(31)=2.75, p<0.01] (Fig. 2A). Even though a similar tendency was found in the other two conditions, there were no significant differences in RTs between TDC and ASD children in both M and MM conditions. Participants with ASD showed significantly lower accuracy than TDC only in the MM condition [t(31)=2.63, p<0.05] (Fig. 2B). There were no significant differences in accuracy between the two groups in N and M conditions.
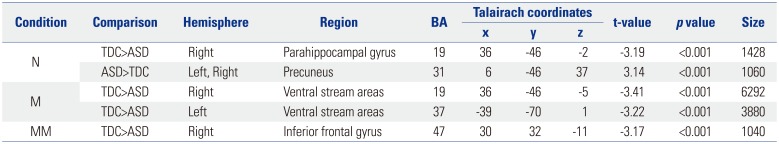
We noted a significant group×condition interaction in the right medial frontal gyrus [MFG; F(2, 93)=6.92, p<0.001] (Supplementary Fig. 1, only online). A main effect of group was significant in the right parahippocampal gyrus [PG; F(1, 93)=10.59, p<0.001] (Supplementary Fig. 2A, only online), and the main effect of condition was significant in several brain regions (Supplementary Table 1, Supplementary Fig. 2B, only online). To assess group differences, we conducted simple effects analysis in all three conditions (Table 2). As shown in Fig. 3, TDC showed a stronger response in the right PG and a weaker response in the precuneus than participants with ASD in the N condition [t(31)=3.19, p<0.001; t(31)=3.14, p<0.001, respectively]. In the M condition, TDC showed a stronger response in the bilateral ventral stream areas [t(31)=3.41, p<0.001 for right hemisphere; t(31)=3.22, p< 0.001 for left hemisphere]. In the MM condition, TDC showed a stronger response in the right inferior frontal gyrus [IFG; t(31)= 3.17, p<0.001).
The aim of the present study was to determine the behavioral and neural differences between children with ASD and TDC in a social language task using Korean idioms. In the MM condition, as we expected, children with ASD showed significantly decreased right inferior frontal gyrus (RIFG) activity, compared with TDC. Previous studies have suggested that the IFG is a component of the mirror neuron system, which is a core structure related to empathy and inference of other's mental states.222324 For fluent social communication, it is essential to understand various intonation, facial expressions, and pragmatic language, such as irony, metaphorical understanding, and idiomatic phrases. In other words, idiom comprehension plays an important role in understanding social language.812 People acquire social language naturally by observing others and mimicking their speech. Therefore, deficits in inference and empathy regarding other's mental states may cause impairments in comprehension of social language. Contrary to our result, increased activity in the RIFG of individuals with ASD relative to TDC was observed in a previous study that incorporated a social language task. This can be explained by a compensatory mechanism to overcome difficulties in integrating contextual cues with given sentences.2526 A previous neuroimaging study revealed activation in several right-hemisphere brain regions during language comprehension.27 Neural activity in the right hemisphere is thought to be associated with higher-level language processing, for example interpretation of ambiguous sentences.28293031
In our results, participants with ASD showed both lower performance accuracy and decreased RIFG activity, compared to TDC, when interpreting idioms with mismatched images. The deficient performance of participants with ASD in contextual language comprehension has been shown in other previous studies.2526 For people with ASD, it is difficult to interpret sentences according to context, such as idioms with matched images, and a compensatory mechanism is needed to overcome them, which seems to be related to activation of the right hemisphere. This discrepancy can be explained as an impaired compensatory mechanism for high-level language processing, such as idiom comprehension, in individuals with ASD; consequently, children with ASD showed significantly lower accuracy than TDC in the MM condition in this study due to failed compensatory processing.
In the M condition, children with ASD showed less activity in the bilateral ventral stream area (VSA) compared with TDC, although behavioral performance of the two groups did not differ significantly. The VSA is known as a visual word-form area that is associated with expertise in visual reading.323334 This cortical area responds preferentially to letter strings than to other categories of stimuli, such as faces or objects.35 In particular, the left VSA shows lateralized neural activity in visual word-recognition, and the right VSA plays a compensatory role.36 Based on these findings, we assume that children with ASD have a neural unique mechanism by which they process pragmatic language, especially for visual word-recognition.
Unexpectedly, in the N condition, participants with ASD showed lower activation of the bilateral precuneus and higher activation of the right PG, and there were no significant differences in behavioral accuracy between the two groups. In a previous study, functional connectivity between the right anterior insula and precuneus was decreased in participants with ASD during deictic shifting.37 Another study showed increased activation of the bilateral PG in high-functioning ASD during active or passive sentence comprehension.38 Initially, we expected that there would be no differences between groups in this condition because the sentences in the N condition could be comprehended literally with the picture provided. However, we found different neural activity in the two groups in several regions related to pragmatic language comprehension. These results likely reflect the aberrant language processing patterns of ASD.
Interestingly, children with ASD exhibited significantly faster RTs than TDC in the N condition. Further, we also found similar tendencies in the other two conditions. To understand idioms, it is necessary to integrate the idiomatic expression and social context in order to determine the speaker's true intentions. Response inhibition is a process that automatic response is withheld when a stop signal is detected; this effortful process is required for idiom comprehending not to interpret the idiom in an overly literal manner. The RIFG has been reported to play an important role in response inhibition.3940 In the present study, the decreased neural activity in the RIFG and the fast RTs that were shown by the participants with ASD can be explained as impaired response inhibition in this population.
There are several potential limitations to this study. First, the stimuli in the KASLAT task consisted of visual sentences and pictures. When people communicate reciprocally, a variety of verbal and non-verbal elements are involved, such as voice, accent, gestures, and facial expressions. However, there are technical limitations to the creation of stimuli that reflect real conversations. Second, the number of participants was limited and their gender ratio differed between the groups. The higher rate of ASD diagnosis in males than females is one possible reason for the gender discrepancy in our study. Finally, we recruited only high functioning children with ASD for this study; therefore, our results cannot be generalized to all individuals with ASD. To elaborate the neurobiological mechanisms of pragmatic language impairment in ASD would require study using more varied stimuli and larger samples.
In conclusion, we showed that children with ASD are less accurate and have different neural activity than TDC while performing an idiom comprehension task. To the best of our knowledge, this is the first fMRI study to show aberrant neural activity underlying pragmatic language processing in Korean children with ASD. Understanding the linguistic characteristics of children with ASD in social contexts can be used to determine educational interventions and to confirm the therapeutic effects of such interventions.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (2016R1A2B4006737).
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). 5th ed. Arlington (VA): American Psychiatric Association;2013.
2. Caronna EB, Milunsky JM, Tager-Flusberg H. Autism spectrum disorders: clinical and research frontiers. Arch Dis Child. 2008; 93:518–523. PMID: 18305076.

3. Tager-Flusberg H, Paul R, Lord C. Language and communication in autism. In : Volkmar FR, Paul R, Klin A, Cohen D, editors. Handbook of autism and pervasive developmental disorders. 3rd ed. Hoboken (NJ): John Wiley & Sons, Inc;2005. p. 335–364.
4. Knaus TA, Silver AM, Lindgren KA, Hadjikhani N, Tager-Flusberg H. fMRI activation during a language task in adolescents with ASD. J Int Neuropsychol Soc. 2008; 14:967–979. PMID: 18954477.

5. Boucher J. Language development in autism. Int J Pediatr Otorhinolaryngol. 2003; 67(Suppl 1):S159–S163. PMID: 14662187.

6. Boucher J. Research review: structural language in autistic spectrum disorder-characteristics and causes. J Child Psychol Psychiatry. 2012; 53:219–233. PMID: 22188468.
7. Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Philos Trans R Soc Lond B Biol Sci. 2003; 358:303–314. PMID: 12639328.

8. Happé FG. Communicative competence and theory of mind in autism: a test of relevance theory. Cognition. 1993; 48:101–119. PMID: 8243028.

9. Colle L, Baron-Cohen S, Hill J. Do children with autism have a theory of mind? A non-verbal test of autism vs. specific language impairment. J Autism Dev Disord. 2007; 37:716–723. PMID: 16977496.

10. Groen WB, Zwiers MP, van der Gaag RJ, Buitelaar JK. The phenotype and neural correlates of language in autism: an integrative review. Neurosci Biobehav Rev. 2008; 32:1416–1425. PMID: 18562003.

11. Levorato MC, Cacciari C. Children's comprehension and production of idioms: the role of context and familiarity. J Child Lang. 1992; 19:415–433. PMID: 1527209.

12. Lee SB, Song SH, Ham JH, Song DH, Cheon KA. Idiom comprehension deficits in high-functioning autism spectrum disorder using a Korean autism social language task. Yonsei Med J. 2015; 56:1613–1618. PMID: 26446644.

13. Lo YC, Chou TL, Fan LY, Gau SS, Chiu YN, Tseng WY. Altered structure-function relations of semantic processing in youths with high-functioning autism: a combined diffusion and functional MRI study. Autism Res. 2013; 6:561–570. PMID: 23853172.

14. Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005; 23:125–141. PMID: 15749240.

15. Siegal M, Blades M. Language and auditory processing in autism. Trends Cogn Sci. 2003; 7:378–380. PMID: 12963465.

16. Happé F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, et al. 'Theory of mind' in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996; 8:197–201. PMID: 9051780.

17. Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002; 125(Pt 8):1839–1849. PMID: 12135974.

18. Kim YS, Leventhal BL, Koh YJ, Fombonne E, Laska E, Lim EC, et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011; 168:904–912. PMID: 21558103.

19. Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994; 24:659–685. PMID: 7814313.

20. Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000; 30:205–223. PMID: 11055457.
21. Cheon KA, Park JI, Koh YJ, Song J, Hong HJ, Kim YK, et al. The social responsiveness scale in relation to DSM IV and DSM5 ASD in Korean children. Autism Res. 2016; 9:970–980. PMID: 27604989.

22. Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003; 100:5497–5502. PMID: 12682281.

23. Seitz RJ, Schäfer R, Scherfeld D, Friederichs S, Popp K, Wittsack HJ, et al. Valuating other people’s emotional face expression: a combined functional magnetic resonance imaging and electroencephalography study. Neuroscience. 2008; 152:713–722. PMID: 18313858.

24. Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009; 132(Pt 3):617–627. PMID: 18971202.

25. Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006; 129(Pt 4):932–943. PMID: 16481375.

26. Tesink CM, Buitelaar JK, Petersson KM, van der Gaag RJ, Kan CC, Tendolkar I, et al. Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain. 2009; 132(Pt 7):1941–1952. PMID: 19423680.

27. Tesink CM, Petersson KM, van Berkum JJ, van den Brink D, Buitelaar JK, Hagoort P. Unification of speaker and meaning in language comprehension: an FMRI study. J Cogn Neurosci. 2009; 21:2085–2099. PMID: 19016606.

28. St George M, Kutas M, Martinez A, Sereno MI. Semantic integration in reading: engagement of the right hemisphere during discourse processing. Brain. 1999; 122(Pt 7):1317–1325. PMID: 10388797.

29. Rodd JM, Davis MH, Johnsrude IS. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cereb Cortex. 2005; 15:1261–1269. PMID: 15635062.

30. Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. Neuroimage. 2005; 25:1002–1015. PMID: 15809000.

31. Zempleni MZ, Renken R, Hoeks JC, Hoogduin JM, Stowe LA. Semantic ambiguity processing in sentence context: evidence from event-related fMRI. Neuroimage. 2007; 34:1270–1279. PMID: 17142061.

32. McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003; 7:293–299. PMID: 12860187.

33. Shaywitz SE, Shaywitz BA. Paying attention to reading: the neurobiology of reading and dyslexia. Dev Psychopathol. 2008; 20:1329–1349. PMID: 18838044.

34. Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, et al. How learning to read changes the cortical networks for vision and language. Science. 2010; 330:1359–1364. PMID: 21071632.

35. Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. Neuroimage. 2004; 22:466–476. PMID: 15110040.

36. Cohen L, Lehéricy S, Henry C, Bourgeois M, Larroque C, Sainte-Rose C, et al. Learning to read without a left occipital lobe: right-hemispheric shift of visual word form area. Ann Neurol. 2004; 56:890–894. PMID: 15562413.

37. Mizuno A, Liu Y, Williams DL, Keller TA, Minshew NJ, Just MA. The neural basis of deictic shifting in linguistic perspective-taking in high-functioning autism. Brain. 2011; 134(Pt 8):2422–2435. PMID: 21733887.

38. Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004; 127(Pt 8):1811–1821. PMID: 15215213.

39. Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010; 50:1313–1319. PMID: 20056157.

40. Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004; 8:170–177. PMID: 15050513.

Supplementary Materials
Supplementary Fig. 1.
Interaction of group×condition. Significant interaction of group×condition was visualized with the statistical significance at corrected p<0.001. ASD, autism spectrum disorder; TDC, typically developing children; MFG, medial frontal gyrus; N, neutral; M, matched; MM, mismatched.
Supplementary Fig. 2.
Main effect of group×condition. Significant effects of (A) group and (B) condition were visualized with the statistical significance at corrected p<0.001. Detailed areas were shown in Supplementary Table 1 (only online).
Fig. 1
Paradigm design and example of the stimuli used in the KASLAT task. (A) This smells weird. This sentence has a similar meaning to the English expression, “I smell a rat.” Literally, these expressions indicate an awkward smell. However, we understand that something suspicious or unreliable is going on in the social context. (B) Father became a pickled onion. This metaphorically implies an exhausted person. (C) He is digging his own grave. This is a Korean proverb that is used when someone refers to doing something stupid or making mistakes. (D) Crying fist. It refers to a person suffering from extreme anger or frustration. (E) Sit with your two legs stretched forward. (F) She shouted from the top of the mountain. s, sec; KASLAT, Korean Autism Social Language Task. N, neutral; M, matched; MM, mismatched.

Fig. 2
Mean RT and accuracy for KASLAT task in the ASD and TDC groups. (A) The RTs (ms) and (B) the percentage of the accuracy of behavior responses were calculated from recorded answers in each condition. Differences were considered statistically significant for *p<0.01, †p<0.05. RT, reaction time; ASD, autism spectrum disorder; TDC, typically developing children; N, neutral; M, matched; MM, mismatched; KASLAT, Korean Autism Social Language Task.

Fig. 3
Different brain activation maps of ASD and TDC with a KASLAT task. ASD, autism spectrum disorder; TDC, typically developing children; N, neutral; M, matched; MM, mismatched; KASLAT, Korean Autism Social Language Task.
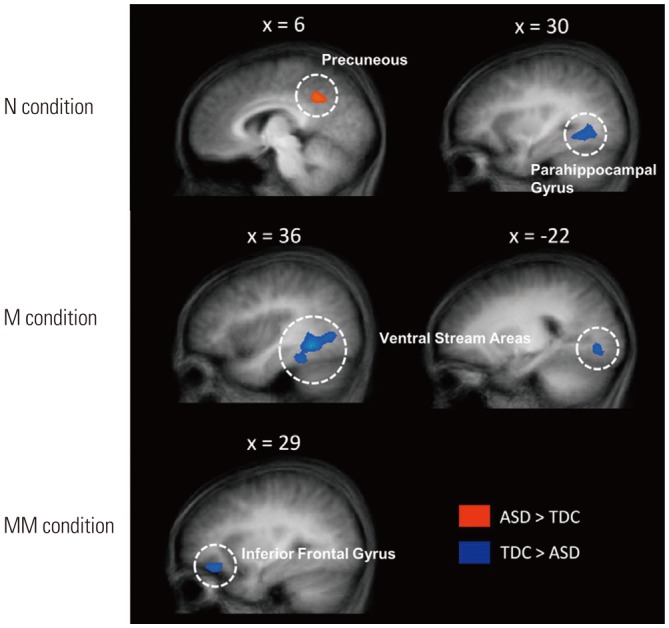
Table 1
Demographic and Neuropsychological Data of the Subjects
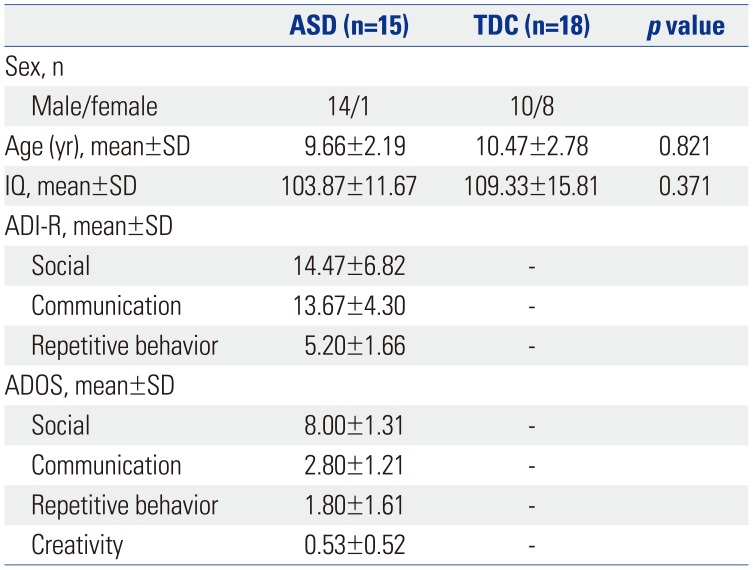
Table 2
Comparison of Activated Brain Areas During the Task in ASD and TDC




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download