INTRODUCTION
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a group of systemic vasculitides encroaching small vessels, from capillaries to intraparenchymal arterioles and venules. AAV consists of three variants, such as microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA).
12 Because AAV may induce damage to major organs, AAV can occasionally be fatal: One year-cumulative patient survival rates were reported to range from 82% to 95% in Western countries
3 and up to 79.1% in Japan.
4 Meanwhile, it was recently reported that 10-year cumulative patient survival rate was 92.8% in Korean patients with AAV.
5
So far, C-reactive protein (CRP)/serum albumin ratio (CAR) has been introduced as an independent predictor of all-cause mortality in cancers, inflammatory diseases and septic conditions.
678 Since, AAV is also an inflammatory disease involving systemic major organs beyond vessels,
12 one could reasonably speculate that CAR might be a predictor of all-cause mortality during follow-up. However, no study has sought to clarify the clinical significance of CAR in predicting all-cause mortality in AAV patients. Hence, in this study, we investigated whether CAR could be an independent predictor of all-cause mortality in 170 patients with AAV.
DISCUSSION
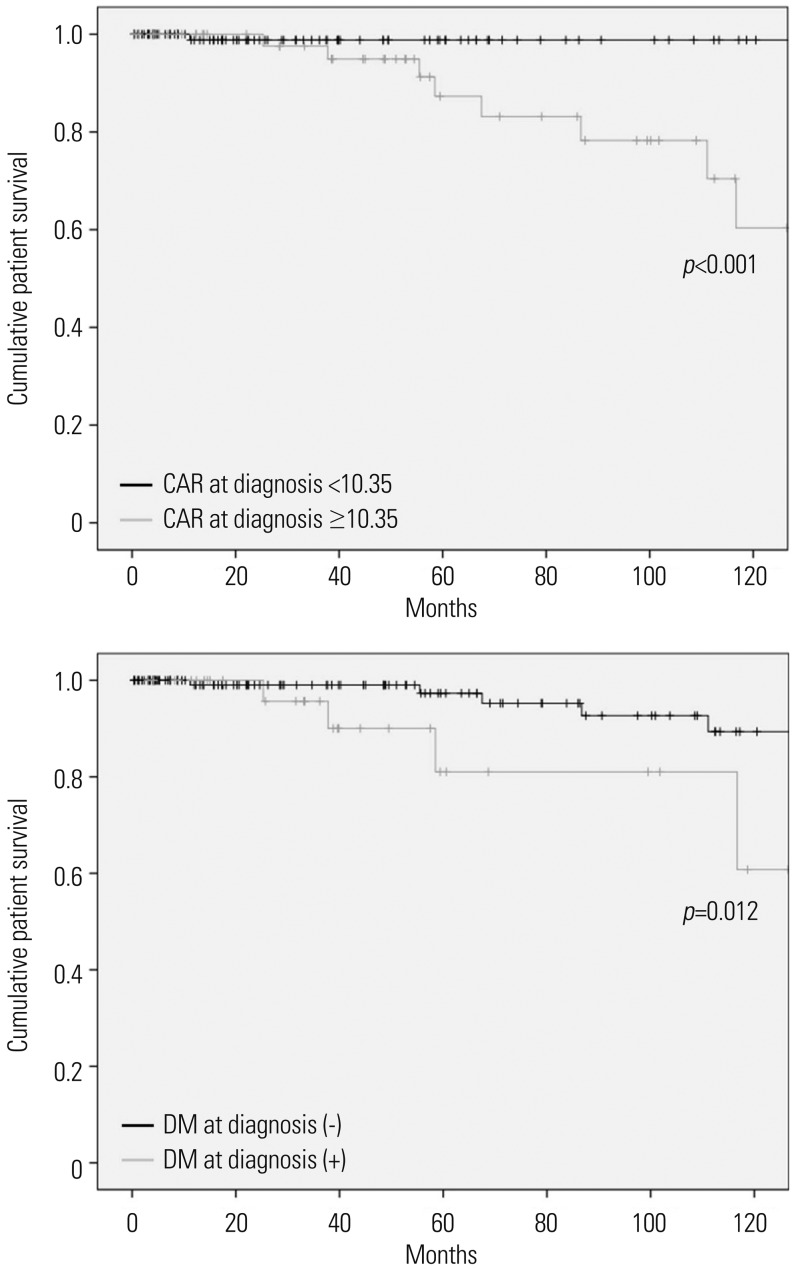
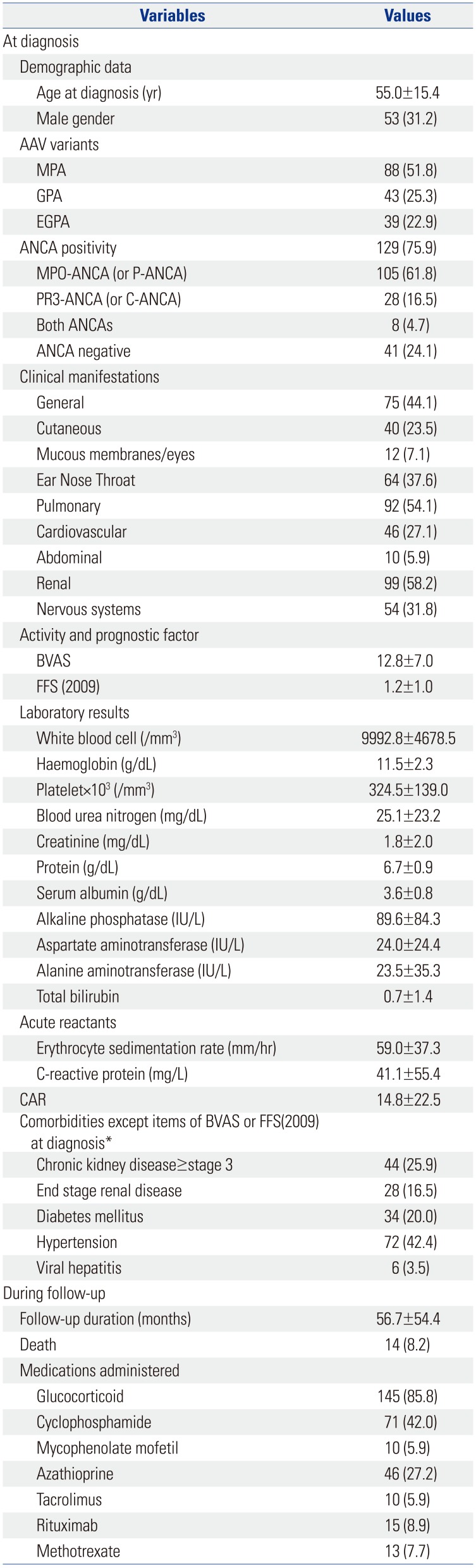
In this study, we investigated whether CAR at diagnosis could predict all-cause mortality during the follow-up of AAV. Also, we demonstrated that CAR at diagnosis is an independent predictor of all-cause mortality comparable to DM, the conventional risk factor of mortality. Also we provided an optimal cut-off of CAR at diagnosis to estimate the risk of all-cause mortality.
How CAR at diagnosis could be an independent predictor of all-cause death in AAV patients could be explained as follows: CRP is closely correlated with interleukin (IL)-6. Chronic inflammation enhances the production of IL-6, which, in turn, promotes the production of CRP in the liver, leading to an increase in CRP level in the peripheral circulation.
11 Meanwhile, IL-6 down-regulates or inhibits the production of albumin in the liver, resulting in a decrease in serum albumin concentration.
12 Accordingly, inflammatory burden can affect CAR via two different directions (increasing CRP and decreasing serum albumin), unlike CRP or serum albumin alone. Thus, CAR with two variables affected by inflammatory burden in AAV could be a more stable and reliable index to presuppose all-cause mortality, compared to CRP or serum albumin alone in AAV patients.
Here we provide two hypotheses regarding the connection between the inflammatory burdens at diagnosis and all-cause mortality in AAV patients. First, a large inflammatory burdens at diagnosis may provoke immunosuppressive drug-refractoriness or cause a situation where patients should take larger doses of immunosuppressive drugs or have a longer period to receive them. This situation may exacerbate the risk of infection and cancer development.
13 Second, a large inflammatory burden may reflect ready-made organ-damage at diagnosis and, furthermore, may result in end stage damage to various major organs during follow-up, such as diffuse alveolar haemorrhage and end stage renal disease.
9 This situation may aggravate the systemic complications of organ-damages by AAV, leading to an increase in all-cause mortality. In this study, the most common cause of death was serious infection in 12 patients [pneumonia (n=7), bacterial sepsis (n=3), influenza infection (n=1), and biliary infection (n=1)]. Two patients died of AAV-related complication [diffuse alveolar haemorrhage (n=1) and cardiac arrest due to cardiomyopathy (n=1)]. This result might support to our hypotheses.
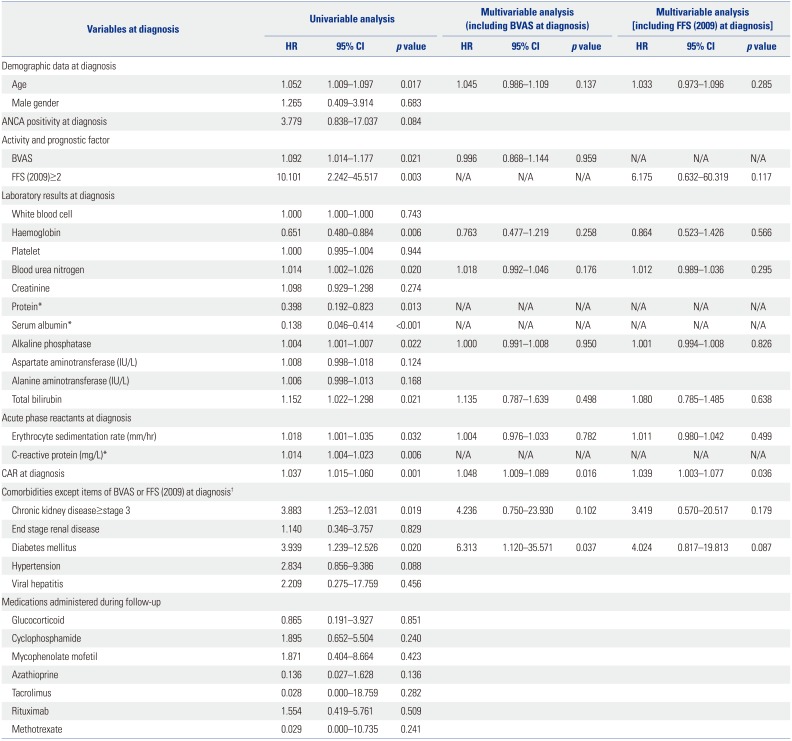
In patients with AAV, infection, cardiovascular damage, malignancies, CKD, high BVAS, low haemoglobin, and immunosuppressive drugs were reported to be associated with all-cause mortality.
14 Meanwhile, age, male gender, DM, and hypertension are also considered as traditional risk factors of all-cause mortality in the general population.
15 Recently, in Korean patients with AAV, it was reported that FFS (2009) at diagnosis ≥2 was associated with mortality.
5 Accordingly, we included initial BVAS and FFS (2009) at diagnosis ≥2 in multivariable Cox hazard model analysis along with both AAV-related and traditional risk factors of all-cause mortality. In univariable analysis, almost all the risk factors exhibited predictive potential for mortality, similar to a previous study.
14 However, in both multivariable Cox hazards model analyses with either initial BVAS or FFS (2009) at diagnosis ≥2, only CAR at diagnosis and DM were identified as an independent predictor of all-cause of mortality. Unlike the previous study investigating whether the initial clinical features and medications administered could predict all-cause mortality,
5 this study evaluated the clinical implications of CAR among clinical and laboratory variables, and so there might be a subtle difference in the results between the two studies.
Although no predictive value for all-cause mortality was witnessed in ANCA positivity (
p=0.084) or hypertension (
p=0.088) in the univariable Cox hazards model analysis in this study, ANCA positivity is one of AAV-related risk factors for serious outcomes,
16 and furthermore, hypertension is a well-known conventional risk factor for mortality in the general population.
15 Thus, to minimise the effect of two confounding factors, we included ANCA positivity and hypertension in the univariable Cox hazard model analysis. Regardless of ANCA positivity and hypertension, only CAR exhibited statistical significance among variables when applying either BVAS at diagnosis or FFS (2009) at diagnosis ≥2 in the model (HR 1.050,
p=0.025, and HR 1.042,
p=0.039) (
Supplementary Table 1, only online).
Our study has an advantage in that we first proved the clinical significance of CAR at diagnosis in predicting all-cause of mortality and furthermore, provided an optimal cut-off of CAR at diagnosis to estimate the risk of all-cause mortality. However, this study also has several limitations. First, as this study was designed as a retrospective study, we could not strictly control for confounding factors, including undocumented histories of medications, nutritional status related the level of serum albumin, and comorbidities. Second, as this study was conducted in a single centre, the number of deceased patients was too small to perform sub-group analysis based on the causes of mortality. Third, we could not use the cut-off of CAR in the multivariate Cox hazard model analysis to presuppose allcause mortality due to the small statistical power. This study may be a pilot study to first investigate the clinical implication of CAR in AAV patients. We believe that future prospective and multi-centric studies will validate the clinical implications of our results, and they will provide a clear optimal cut-off of CAR to predict all-cause mortality in AAV patients for use in real clinical settings. In conclusion, CAR at diagnosis can be an independent predictor of all-cause mortality, comparable to DM, the conventional risk factor of mortality.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download