Abstract
Purpose
Anti-vascular endothelial growth factor (VEGF) agents have been used for the last 10 years, but their safety profile, including cytotoxicity against various ocular cells such as retinal pigment epithelial (RPE) cells, remains a serious concern. Safety studies of VEGF agents conducted to date have primarily relied on healthy RPE cells. In this study, we assessed the safety of three anti-VEGF agents, namely, ranibizumab, bevacizumab, and aflibercept, on senescent RPE cells.
Methods
Senescent human induced pluripotent stem cell-derived RPE cells were generated by continuous replication and confirmed with senescence biomarkers. The viability, proliferation, protein expression, and phagocytosis of the senescent RPE cells were characterized 3 days after anti-VEGF treatment with clinical doses of ranibizumab, bevacizumab, or aflibercept.
Results
Clinical doses of ranibizumab, bevacizumab, or aflibercept did not decrease the viability or alter proliferation of senescent RPE cells. In addition, the anti-VEGF agents did not induce additional senescence, impair the protein expression of zonula occludens-1 and RPE65, or reduce the phagocytosis capacity of senescent RPE cells.
Age-related macular degeneration (AMD) is the leading cause of irreversible vision loss in patients older than 50 years of age in developed countries [1]. Clinically, AMD is classified into ‘dry’ and ‘wet’ forms. The dry form of AMD is more common than the wet form, and accounts for approximately 80% to 90% of AMD cases. Whereas dry AMD progresses more slowly than wet AMD and often does not result in vision loss, wet AMD is characterized by choroidal neovascularization that originates from the choriocapillaris. Wet AMD often progresses into the subretinal space through Bruch's membrane and tends to bleed and leak, ultimately leading to irreversible damage to photoreceptor cells if left untreated. Thus, most of the cases of significant visual loss from AMD occur in patients with the wet form of the disease. The cause and progression of choroidal neovascularization in patients with wet AMD is poorly understood, but vascular endothelial growth factor (VEGF) has been implicated as the most important factor promoting neovascularization [23].
Senescence of retinal pigment epithelial (RPE) cells has been hypothesized to play a critical role in the pathology of AMD. Chronic inflammation, complement activation, and angiogenesis, which are all involved in the development of AMD, are directly or indirectly associated with RPE cell senescence [4]. Marazita et al. [5] reported that premature senescence of ARPE-19 cells leads to dysregulated e xpression o f VEGF a nd c omplement f actor H, which are involved in the pathogenesis of AMD. In addition, senescent cells may promote an inflammatory response [6]. RPE cell senescence is also consistent with the majority of the clinical signs of AMD. For example, liposomal proteolysis is dysfunctional in senescent RPE cells, which may lead to improper processing in the photoreceptor outer segments (POSs) and formation of deposits known as drusen.
Several clinical trials have established the efficacy of anti-VEGF agents for the treatment of wet AMD including ranibizumab [78], bevacizumab [910], and af libercept [1112]. These anti-VEGF agents provide unprecedented visual benefits for patients with wet AMD; however, their safety remains a concern. Many in vitro studies have reported that ranibizumab, bevacizumab, and aflibercept at clinical dosages have little or no significant cytotoxicity on RPE cells [13141516171819]. Moreover, the use of anti-VEGF agents appears to be safe in actual clinical practice. However, some recent clinical studies have reported that intensive and continuous therapy with anti-VEGF agents is associated with an increased incidence of RPE cell atrophy and the lesion size of geographic atrophy [2021].
Previous in vitro studies have primarily relied on healthy RPE cells to evaluate the safety of anti-VEGF agents [13141516171819]. However, the RPE cells of patients with wet AMD can be assumed to be in a senescent state, and thus the safety of anti-VEGF agents specifically on senescent RPE cells requires further investigation. To date, there have been no studies on the effects of a nti-VEGF agents on senescent RPE cells. Furthermore, it has not been definitively established whether senescent RPE cells are more negatively affected by anti-VEGF agents compared to healthy RPE cells. Therefore, the purpose of the current study was to determine the effects of ranibizumab, bevacizumab, and aflibercept on senescent human RPE cells.
Human induced pluripotent stem cell (hiPSC) lines were obtained from the RIKEN BioResource Center (Ibaraki, Japan) and the American Type Culture Collection (Manassas, VA, USA). Cells were cultured on Matrigel (BD Biosciences, San Diego, CA, USA) in feeder-free conditions. The differentiation of RPE cells from hiPSCs was performed as previously described [22]. Briefly, embryoid bodies were formed and cultured on ultra-low attachment dishes in neural induction medium for 6 days. Embryoid bodies were seeded onto Matrigel-coated plates and cultured in RPE cell medium for 4 weeks. The pigmented clusters were then mechanically dissected and cultured in monolayers.
A small number of hiPSC-derived RPE cells (1 × 102 cells) were seeded onto Matrigel-coated 12-well plates and cultured (passage 0). Shortly after reaching 100% confluency, subculturing was performed using the same number (1 × 102) of hiPSC-derived RPE cells. Some of the RPE cells remained in the cultivating plates and were used without subsequent subculture (non-passaged cells) for the purpose of comparison with senescent RPE cells. For other RPE cells, subculturing was repeated serially at least 3 or 6 times. In this way, hiPSC-derived RPE cells were forced to undergo replication exhaustion by continuous mitosis (serial passaging of cells) for the purpose of establishing cellular senescence.
Ranibizumab (Lucentis; Genentech, San Francisco, CA, USA), bevacizumab (Avastin, Genentech), and aflibercept (Eylea; Regeneron, Tarrytown, NY, USA) were diluted in culture media to concentrations equivalent to the doses used in clinical practice. The clinical dose was calculated by assuming that the amount of intravitreally injected anti-VEGF agent was diluted equally throughout the 4-mL average volume of human vitreous. Ranibizumab (10 mg/mL), bevacizumab (25 mg/mL), and aflibercept (40 mg/mL) were used at doses of 0.3 mg, 1.25 mg, and 2.0 mg per 4 mL culture medium, respectively. In each experiment, senescent hiPSC-derived RPE cells were cultivated in culture medium mixed with ranibizumab, bevacizumab, or aflibercept for 72 hours.
Senescence of hiPSC-derived RPE cells was examined using the senescence-associated β-galactosidase (SA-β-gal) staining kit (Cell Signaling Technology, Beverly, MA, USA) according to the manufacture's instructions. SA-β-gal-stained RPE cells were photographed at ×200 magnification. The percentage of SA-β-gal-stained cells was evaluated by quantifying a minimum of 500 cells in 5 randomly selected microscopic fields. The values obtained from at least three independent experiments were averaged and the data are presented as the mean ± standard deviation.
Total RNA was prepared from ARPE-19 cells, hiPSC-derived RPE cells, and adult human RPE tissue using a RNeasy kit (Qiagen, Hilden, Germany) with on-column DNase digestion. Two microgram of total RNA was reverse-transcribed using Superscript III (Invitrogen, Carlsbad, CA, USA). Polymerase chain reaction amplification was performed using specific primer pairs as follows: Rpe65, 5′- ACATATGCGTATGGACTTGG -3′ and 5′- GAACAGTCCATGAAAGGTGA -3′; Mitf, 5′- AAAGAACTTGAAAACCGACA -3′ and 5′- ACAGGTTAGGTCTGCATGAT -3′; Cralbp, 5′- TGCTCAGAGGCTATGTGAAT -3′ and 5′- A ATATG C C TG CA AG AT C T CA -3′; Ty r p1, 5′- AAGAAAAGAACCACTTTGTCC -3′ and 5′- TGAGAGAAATCCACTTCACC -3′; Tyr, 5′- AGAGACGACTCTTGGTGAGA -3′ and 5′- AGTGCATCCATTGACACATA -3′; Pedf, 5′- AGCTCGCCAGGTCCACAAAG -3′ and 5′- TGGGCAATCTTGCAGCTGAG- 3′; Pedf, 5′- AGCTCGCCAGGTCCACAAAG -3′ and 5′- TGGGCAATCTTGCAGCTGAG-3′; Vegf, 5′- AGAAATCCCGGTATAAGTCC -3′ and 5′- ATCTGCAAGTACGTTCGTTT -3′; and Gapdh, 5′ -GTCAGTGGTGGACCTGACCT -3′ and 5′- CACCACCCTGTTGCTGTAGC -3′.
Cells were lysed in lysis buffer containing 25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 10 mM sodium pyrophosphate, 10 mM β-glycerophosphate, 1 mM sodium orthovanadate, 10% glycerol, and protease inhibitors (Roche, Basel, Switzerland). Each protein sample was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes. After blocking, blots were sequentially incubated with a primary antibody and peroxidase-conjugated secondary antibody. Blots were developed by enhanced chemiluminescence reagent (Bio-Rad, Hercules, CA, USA), and the intensity of bands was quantified using Image Lab software (Bio-Rad). The following primary antibodies were used: rabbit anti-p21 (C-19; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-p27 (F-8; Santa Cruz Biotechnologies), and mouse anti-p53 (DO-1; Santa Cruz Biotechnologies).
The viability of hiPSC-derived RPE cells was evaluated using a 4-(3-(4-iodophenyl)-2(4-nitrophenyl)-2H-5-tetrazolio)-1,3-benzene disulfonate (WST-1) assay (Roche). Senescent hiPSC-derived RPE cells were plated in 96-well microplates at a density of 1.5 × 103 cells/well. Senescent RPE cells were t reated with aflibercept, ranibizumab, or bevacizumab and then incubated for 72 hours. The WST-1 assay solution was added (10 µL/well), after which the cells were incubated for 2 hours at 37℃ and the absorbance was measured at 450 nm using a microplate reader (Model 680, Bio-Rad). All experiments were performed in triplicate. The absorbance values were expressed as a percentage of the controls representing 100% cell viability.
The effects of a nti-VEGF agents on cellular proliferation was evaluated by quantification of 5-ethynyl-2-deoxyuridine (EdU) incorporation into genomic DNA using the Click-iT EdU cell proliferation assay kit (Invitrogen). Briefly, RPE cells were cultured overnight in medium containing EdU at a final concentration of 10 µM. Next, RPE cells were fixed in 4% paraformaldehyde at room temperature, rinsed, and incubated with Click-iT EdU reaction cocktail for 30 minutes in the dark. The RPE cells were then photographed using a fluorescence microscope with an AxioCam camera with Axio Imager software (Zeiss, Oberkochen, Germany). The number of EdU-positive RPE cells was counted in five randomly selected microscopic fields. The percentage of EdU-positive cells was obtained by dividing the number of positive cells by the total number of cells present in the same microscopic fields. Data are presented as the mean ± standard deviation of at least three independent experiments.
Induction of apoptosis in senescent RPE cells treated with anti-VEGF agents for 72 hours was analyzed using an in situ cell death detection kit (Roche) according to the manufacturer's recommended protocol. Senescent RPE cells were fixed in 4% paraformaldehyde and permeabilized in 0.1% Triton-X 100 (Sigma-Aldrich, St. Louis, MO, USA). After washing with phosphate-buffered saline, the RPE cells were incubated with the terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) reaction mixture for 1 hour at 37℃ in the dark. TUNEL-positive RPE cells were calculated as the percentage of total nuclei. Senescent RPE cells treated with 50 µM hydrogen peroxide (H2O2) were used a s a positive control f or the TUNEL assay. Experiments we re repeated three times.
Immunofluorescence detection of zonula occludens-1 (ZO-1) and retinoid isomerohydrolase (RPE65) was used to determine whether the anti-VEGF agents altered the expression of mature RPE cell markers. Senescent RPE cells were treated with the aforementioned concentrations of bevacizumab, ranibizumab, or aflibercept for 72 hours. Immunofluorescence was performed using antibodies to ZO-1 (Invitrogen) diluted to 1 : 250 and RPE65 (Novus, Littleton, CO, USA) diluted to 1 : 500. Images were obtained with a fluorescence microscope with an AxioCam camera (Zeiss).
Phagocytosis assays were performed as previously described [23]. Briefly, POSs were prepared from the retinas of donated human eyes. Senescent RPE cells treated with anti-VEGF agents were incubated with 10 µg POS at 37℃ in 5% CO2 for 6 hours, and were then washed to remove external POS after which the RPE cells were fixed. The cells were then immunostained with mouse monoclonal anti-rhodopsin antibody. Photomicrographs of the total POS uptake were obtained using a fluorescence confocal microscope (LSM5 PASCAL, Zeiss). Internalized POSs were documented in the photographs and the percentage of RPE cells with an internalized POS was calculated. Phagocytosis assays were performed in triplicate.
We first generated RPE cells from hiPSCs to investigate the safety of anti-VEGF agents on senescent human RPE cells. The hiPSC-derived RPE cells expressed RPE cell markers as detected by reverse transcriptase-polymerase chain reaction (Fig. 1A). Additionally, the pigmented cells exhibited a hexagonal monolayer RPE cell phenotype (Fig. 1B). Senescent RPE cells from hiPSC-derived RPE cells were developed as an in vitro model for this study. Cellular senescence of hiPSC-derived RPE cells was effectively accelerated by continuous mitosis and established after serial passaging (Fig. 1B). We confirmed cellular senescence based on the significant level of SA-β-gal staining (Fig. 1C). The percentage of SA-β-gal-positive RPE cells was 81.7 ± 5.77% in serially passaged cells, whereas it was 11.0 ± 3.60% and 24.1 ± 3.66% in passage 0 and non-passaged RPE cells, respectively. We next determined the mRNA expression of RPE cell markers in serially passaged RPE cells. Although some markers (RPE65, pigment epithelium-derived factor, and VEGF) were present at low levels compared to those in passage 0 or non-passaged RPE cells, all RPE cell markers were expressed (Fig. 1D). Other biomarkers of senescence including p21, p27, and p53 were also significantly expressed in serially passaged RPE cells compared to passage 0 or non-passaged RPE cells (Fig. 1E). Therefore, serial passaging of RPE cells was considered to be a reliable method for generating senescent RPE cells.
Induction of apoptosis in senescent RPE cells treated with clinical concentrations of ranibizumab, bevacizumab, or aflibercept was determined by the TUNEL assay (Fig. 2A). A positive TUNEL reaction was not observed in senescent RPE cells treated with any of the three anti-VEGF agents, whereas many TUNEL-positive cells were present in H2O2-treated RPE cells, which served as a positive control (Fig. 2B). Similarly, the WST-1 assay showed that treatment with ranibizumab, bevacizumab, or aflibercept did not result in any significant cytotoxicity in senescent RPE cells (p > 0.05) (Fig. 2C). As expected, the viability of senescent RPE cells was significantly reduced by H2O2 treatment. Pigmentation is one of the characteristics of RPE cells, and there was no significant change in pigmentation of senescent RPE cells after treatment with ranibizumab, bevacizumab or aflibercept. On the other hand, H2O2 treatment significantly reduced the pigmentation of senescent RPE cells (Fig. 2D).
To investigate the effects of anti-VEGF agents on senescent RPE cell proliferation, we performed cell proliferation assays using the EdU reagent. The percentage of EdU-positive RPE cells was determined by counting the total number of RPE cells and EdU-positive RPE cells in selected areas. There were no significant differences in the percentage of EdU-positive RPE cells between naïve senescent RPE cells and senescent RPE cells treated with anti-VEGF agents (Fig. 3A). Among senescent RPE cells, the basal percentage of EdU-positive cells was 0.18 ± 0.03%, which was not significantly different after treatment with ranibizumab, bevacizumab, and aflibercept, with associated percentages of EdU-positive cells of 0.19 ± 0.04%, 0.15 ± 0.02%, and 0.23 ± 0.03%, respectively (all comparisons, p > 0.05) (Fig. 3B).
To determine whether any of the anti-VEGF agents were capable of accelerating cellular senescence, SA-β-gal staining of senescent RPE cells was assessed after incubation of the cells with ranibizumab, bevacizumab, or aflibercept for 72 hours. None of the anti-VEGF agents significantly changed the number of SA-β-gal-positive RPE cells (Fig. 4A). We found that 81.2 ± 5.44% of the senescent RPE cells stained positively for SA-β-gal. The percentages of SA-β-gal-positive RPE cells were not significantly different after treatment with the anti-VEGF agents ranibizumab, bevacizumab, and aflibercept were 78.8 ± 7.08%, 83.7 ± 5.78%, and 76.2 ± 6.45%, respectively (all comparisons, p > 0.05) (Fig. 4B).
We next evaluated the effects of a nti-VEGF agents on the expression of ZO-1, a tight junction protein, in senescent RPE cells. In a similar manner, we also evaluated the expression of RPE65 with immunofluorescence staining. Senescent RPE cells with or without anti-VEGF treatment exhibited a uniform polygonal shape and continuous ZO-1 membrane staining. None of the anti-VEGF agents ranibizumab, bevacizumab, and aflibercept had a negative effect on ZO-1 expression (Fig. 5A). Similarly, RPE65 staining was noted in senescent RPE cells, and treatment of the senescent RPE cells with ranibizumab, bevacizumab, or aflibercept did not have significant negative effects on its expression (Fig. 5B). Western blot analysis also showed that the expression of ZO-1 or RPE65 was not different among senescent RPE cells treated with any of the anti-VEGF agents (Fig. 5C).
Senescent RPE cells treated with ranibizumab, bevacizumab, or aflibercept did not show decreased phagocytosis of rhodopsin compared to naïve senescent RPE cells (Fig. 6A). The percentages of cells positive for intracellular rhodopsin were 58.2 ± 5.12% in naïve senescent RPE cells, 53.3 ± 4.16% in ranibizumab-treated cells, 54.3 ± 6.24% in bevacizumab-treated cells, and 55.3 ± 4.51% in aflibercept-treated cells (Fig. 6B). There was no difference between senescent RPE cells and anti-VEGF agent-treated senescent RPE cells (all comparisons, p > 0.05).
In the present study, we used senescent RPE cells rather than the more commonly utilized healthy RPE cells to investigate the safety of three different anti-VEGF agents. The majority of patients w ith wet A MD have senescent RPE cells rather than young and healthy RPE cells. Thus, we considered our approach to be significant and unique compared to previous studies designed to assess the safety of anti-VEGF agents, which did not account for the differences between healthy and senescent RPE cells. Our data showed that clinically relevant doses of ranibizumab, bevacizumab, or aflibercept had no deleterious effects on senescent RPE cell viability, as demonstrated by the results of TUNEL, WST-1, and cell proliferation assays. In addition, none of the anti-VEGF agent treatments led to exacerbation of RPE cell senescence. Furthermore, neither RPE phagocytosis nor expression of the proteins ZO-1 or RPE65 were impaired by ranibizumab, bevacizumab, or aflibercept.
The induction of premature cellular senescence can be increased by chronic exposure to cellular stresses such as oxidative stress, DNA damaging agents, or activation of certain oncogenes [24]. Senescence is characterized by changes in cellular morphology, increased SA-β-gal activity, decreased replicative capacity, increased expression of p53, p21, p16, and p27, and accumulation of transcriptionally inactive heterochromatin [25]. In this study, cellular senescence of hiPSC-derived RPE cells was generated by replication exhaustion during continuous cell proliferation. In this process, a small amount of hiPSC-derived RPE cells were transferred to new culture dishes on the first subculture to allow RPE cells to undergo continuous mitosis. The same process was repeated during subsequent subculturing. It is well known that during mitosis, telomeres become shortened because the end of the lagging strand of the chromosome cannot be fully replicated [26]. Although this loss is partly or fully restored by the telomerase enzyme, the resulting damage is thought to be a major biological trigger for senescence [27]. hiPSC-derived RPE cells reportedly undergo rapid telomere shortening and chromosomal damage, along with increased p21 expression [28]. These factors may cause rapid senescence, because hiPSC- derived RPE cells are forced into continuous mitosis. In our study, hiPSC-derived RPE cells undergoing continuous mitosis were strongly positive for SA-β-gal activity. In addition, other biomarkers of senescence were expressed, including p53 and the cyclin-dependent kinase inhibitors p16, p21, and p27. Based on these results, we concluded that a state of premature cellular senescence was fully established by our approach of serial passaging.
We observed no change in cell viability after exposing senescent RPE cells to clinical doses of bevacizumab, ranibizumab, or aflibercept for 72 hours. These results were similar to the findings of previous studies using healthy RPE c ells to a ssess c ell viability a fter drug e xposure, which reported no significant differences in cell viability using different concentrations of anti-VEGF agents [131415161819]. Malik et al. [13] reported the safety of anti-VEGF agents on ARPE-19 cells by assessing cell viability after treatment with ranibizumab, bevacizumab, aflibercept, or ziv-aflibercept. While our results were obtained using clinical doses, previous studies have shown that higher concentrations (10 fold greater than the clinical dose) of bevacizumab, aflibercept, or ziv-aflibercept, but not ranibizumab, significantly decrease ARPE-19 cell viability. Saenz-de-Viteri et al. [18] also reported that different doses of ranibizumab, bevacizumab, or aflibercept do not decrease the viability of ARPE-19 cells, although studies by others have reported that bevacizumab at higher concentrations decreases RPE cell viability [19].
There have been some concerns about VEGF depletion toxicity. Using a murine model, Ford et al. [29] reported that systemic VEGF neutralization by bevacizumab leads to transient RPE degenerative change such as vacuolization and detachment from POSs. Kurihara et al. [30] reported that the choriocapillaris rapidly disappears following RPE-specific deletion of the Vegfa gene in adult mice, leading to the death of cone photoreceptors. With respect to long-term clinical usage of anti-VEGF agents, there have also been several controversial reports on the high incidence of retinal geographic atrophy by RPE damage and photoreceptor cell death [2131].
With respect to phagocytosis, our results showed that senescent RPE cells treated with anti-VEGF agents had very slightly diminished phagocytosis capacity, but also that there was no statistically significant difference in overall phagocytic function between naïve senescent RPE cells and anti-VEGF agent-treated cells. It is not certain whether the anti-VEGF agents affected the phagocytic activity of senescent RPE cells or whether the 72-hour treatment of anti-VEGF agents was too short to identify any deleterious effects on RPE cell function. Notably, a study using a clinical dose of aflibercept in primary porcine RPE cells reported a decrease in phagocytic capacity of RPE cells but without evidence of cytotoxicity [17]. Further investigations will be needed to evaluate this discrepancy, because even small changes in the phagocytic capacity of RPE cells may lead to long-term adverse effects on photoreceptors and RPE cells. Large clinical trials have already reported that atrophy of the RPE is enhanced after treatment with bevacizumab or ranibizumab for two years, which may be especially exacerbated by ranibizumab [2021].
There were some limitations in our study. First, it will be necessary in the future to clarify whether in situ RPE cell senescence truly occurs in patients with AMD, which was an assumption made by this study. Secondly, the characteristics of hiPSC-derived RPE cells are distinct from those of actual human RPE cells. Whether the replicative senescence model used in our study represents the natural aging of RPE cell in AMD was another limitation of our study. Lastly, our results were obtained using in vitro experiments, which cannot be directly compared to in vivo studies, and will require validation in future in vivo or clinical studies.
In conclusion, our in vitro study showed that ranibizumab, bevacizumab, and aflibercept at clinically relevant concentrations did not induce significant cytotoxicity in senescent RPE cells. Based on these findings, we inferred that intravitreal injection of ranibizumab, bevacizumab, or aflibercept is unlikely to cause significant damage to senescent RPE cells, although RPE cells in patients with wet AMD may be compromised by aging or the disease itself. Thus, additional studies are needed to evaluate the effects of anti-VEGF agents on senescent RPE cells with repeated doses and long-term treatments.
Figures and Tables
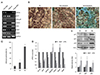 | Fig. 1(A) Expression of retinal pigment epithelial (RPE) cell specific markers in human induced pluripotent stem cell (hiPSC)-derived RPE cells, ARPE-19 cells, and adult RPE tissue. (B) hiPSC-derived RPE cells were stained for senescence-associated b-galactosidase (SA-β-gal) activity. Serially passaged hiPSC-derived RPE cells stained strongly with SA-β-gal. (C) The percentages of SA-β-gal-positive RPE cells at passage 0, without passaging, or after serial passaging. (D) Expression of RPE cell markers. (E) Protein expression of the senescence biomarkers p21, p27, and p53 evaluated by Western blotting. P0 = passage 0; NP = non-passaged; PA = serial passaging. *p < 0.05, **p < 0.01. |
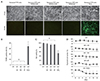 | Fig. 2(A) The terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) staining of senescent retinal pigment epithelial (RPE) cells treated with anti-vascular endothelial growth factor (VEGF) agents. There was no significant increase in TUNEL-positive cells among senescent RPE cells treated with anti-VEGF agents. (B) Percentage of TUNEL-positive senescent RPE cells. (C) The viability of senescent RPE cells treated with anti-VEGF agents was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. (D) The change in pigmentation of senescent RPE cells was evaluated after anti-VEGF treatment. ns = non-specific; sen = senescence; ran = ranibizumab; bev = bevacizumab; afl = aflibercept. *p < 0.05. Bar = 200 µm. |
 | Fig. 3(A) Proliferation of senescent retinal pigment epithelial (RPE) cells treated with anti-vascular endothelial growth factor agents were evaluated using the 5-ethynyl-2-deoxyuridine (EdU) assay. Red dots (EdU-positive cells) represent proliferating RPE cells. (B) Percentage of EdU-positive senescent RPE cells. There were no differences in cell proliferation between naïve senescent RPE cells and anti-vascular endothelial growth factor-treated senescent RPE cells. ns = non-specific; sen = senescence; ran = ranibizumab; bev = bevacizumab; afl = aflibercept. Bar = 200 µm. |
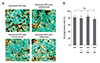 | Fig. 4(A) Effects of anti-vascular endothelial growth factor treatment on senescence of retinal pigment epithelial (RPE) cells. (B) Percentage of senescence-associated-β-galactosidase (SA-β-gal) positive senescent RPE cells. Anti-vascular endothelial growth factor treatment did not cause additional senescence of RPE cells. ns = non-specific; sen = senescence; ran = ranibizumab; bev = bevacizumab; afl = aflibercept. Bar = 200 µm. |
 | Fig. 5(A) Immunofluorescence staining for zonula occludens-1 (ZO-1). (B) Immunofluorescence staining for RPE65. ZO-1 and RPE65 proteins were uniformly expressed regardless of whether senescent retinal pigment epithelial (RPE) cells were treated with anti-vascular endothelial growth factor agents. (C) Western blot assay for the expression of ZO-1 and RPE65 in senescent RPE cells treated with each of anti-vascular endothelial growth factor agents. sen = senescence; ran = ranibizumab; bev = bevacizumab; afl = aflibercept. Bar = 200 µm. |
 | Fig. 6(A) Phagocytosis in senescent retinal pigment epithelial (RPE) cells. Senescent RPE cells were treated with photoreceptor outer segments for 6 hours. Intracellular rhodopsin (red) was detected by immunocytochemistry. The red fluorescence crossing the cell membrane consisted primarily of debris remaining on the cell surface after washing. No difference in the number of rhodopsin positive cells was observed between naïve senescent RPE cells and anti-vascular endothelial growth factor-treated senescent RPE cells. (B) Percentages of rhodopsin-phagocytosing senescent RPE cells. ns = non-specific; sen = senescence; ran = ranibizumab; bev = bevacizumab; afl = aflibercept. |
Notes
References
1. Fine SL, Berger JW, Maguire MG, Ho AC. Age-related macular degeneration. N Engl J Med. 2000; 342:483–492.

2. Kliffen M, Sharma HS, Mooy CM, et al. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997; 81:154–162.

3. Rakic JM, Lambert V, Devy L, et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003; 44:3186–3193.

4. Kozlowski MR. RPE cell senescence: a key contributor to age-related macular degeneration. Med Hypotheses. 2012; 78:505–510.

5. Marazita MC, Dugour A, Marquioni-Ramella MD, et al. Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: implications for age-related macular degeneration. Redox Biol. 2016; 7:78–87.

6. Mishima K, Handa JT, Aotaki-Keen A, et al. Senescence-associated beta-galactosidase histochemistry for the primate eye. Invest Ophthalmol Vis Sci. 1999; 40:1590–1593.
7. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.

8. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.

9. Yoganathan P, Deramo VA, Lai JC, et al. Visual improvement following intravitreal bevacizumab (Avastin) in exudative age-related macular degeneration. Retina. 2006; 26:994–998.

10. Aggio FB, Farah ME, Silva WC, Melo GB. Intravitreal bevacizumab for exudative age-related macular degeneration after multiple treatments. Graefes Arch Clin Exp Ophthalmol. 2007; 245:215–220.

11. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–2548.

12. Talks JS, Lotery AJ, Ghanchi F, et al. First-year visual acuity outcomes of providing aflibercept according to the VIEW study protocol for age-related macular degeneration. Ophthalmology. 2016; 123:337–343.

13. Malik D, Tarek M, Caceres del Carpio J, et al. Safety profiles of anti-VEGF drugs: bevacizumab, ranibizumab, aflibercept and ziv-aflibercept on human retinal pigment epithelium cells in culture. Br J Ophthalmol. 2014; 98 Suppl 1. i11–i16.

14. Schnichels S, Hagemann U, Januschowski K, et al. Comparative toxicity and proliferation testing of aflibercept, bevacizumab and ranibizumab on different ocular cells. Br J Ophthalmol. 2013; 97:917–923.

15. Ammar DA, Mandava N, Kahook MY. The effects of aflibercept on the viability and metabolism of ocular cells in vitro. Retina. 2013; 33:1056–1061.

16. Brar VS, Sharma RK, Murthy RK, Chalam KV. Evaluation of differential toxicity of varying doses of bevacizumab on retinal ganglion cells, retinal pigment epithelial cells, and vascular endothelial growth factor-enriched choroidal endothelial cells. J Ocul Pharmacol Ther. 2009; 25:507–511.

17. Klettner A, Tahmaz N, Dithmer M, et al. Effects of aflibercept on primary RPE cells: toxicity, wound healing, uptake and phagocytosis. Br J Ophthalmol. 2014; 98:1448–1452.

18. Saenz-de-Viteri M, Fernandez-Robredo P, Hernandez M, et al. Single- and repeated-dose toxicity study of bevacizumab, ranibizumab, and aflibercept in ARPE-19 cells under normal and oxidative stress conditions. Biochem Pharmacol. 2016; 103:129–139.

19. Spitzer MS, Wallenfels-Thilo B, Sierra A, et al. Antiproliferative and cytotoxic properties of bevacizumab on different ocular cells. Br J Ophthalmol. 2006; 90:1316–1321.

20. Grunwald JE, Pistilli M, Ying GS, et al. Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2015; 122:809–816.

21. Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014; 121:150–161.
22. Zhu Y, Carido M, Meinhardt A, et al. Three-dimensional neuroepithelial culture from human embryonic stem cells and its use for quantitative conversion to retinal pigment epithelium. PLoS One. 2013; 8:e54552.

23. Lin H, Clegg DO. Integrin alphavbeta5 participates in the binding of photoreceptor rod outer segments during phagocytosis by cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998; 39:1703–1712.
24. Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010; 24:2463–2479.

25. Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013; 32:5129–5143.

26. Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990; 345:458–460.

27. Zhu H, Belcher M, van der Harst P. Healthy aging and disease: role for telomere biology? Clin Sci (Lond). 2011; 120:427–440.

28. Kokkinaki M, Sahibzada N, Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. 2011; 29:825–835.

29. Ford KM, Saint-Geniez M, Walshe T, et al. Expression and role of VEGF in the adult retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011; 52:9478–9487.

30. Kurihara T, Westenskow PD, Bravo S, et al. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest. 2012; 122:4213–4217.

31. Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013; 120:2292–2299.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download