Abstract
Purpose
To evaluate the effectiveness of intravitreal injection of ranibizumab (IVR) in treating diabetic macular edema (DME) with serous retinal detachment (SRD) based on spectral domain optical coherence tomography (SD-OCT) patterns.
Methods
One hundred thirty-four eyes of 134 patients with DME who underwent SD-OCT evaluation were included in this study. We retrospectively analyzed the medical records of patients who received IVR for the treatment of DME. Their eyes were classified into three groups according to the following SD-OCT features: SRD, diffuse retinal thickness and cystoid macular edema. The three groups were compared regarding changes in best-corrected visual acuity and central foveal thickness (CFT) after IVR.
Results
The mean age was 61.4 ± 9.2 years (range, 44 to 81 years). The average length of the follow-up period was 9.4 ± 3.4 months (range, 6 to 24 months). The mean CFT value was significantly reduced in all groups (p < 0.001) after treatment. Increases in best-corrected visual acuity were statistically significant for the diffuse retinal thickness and cystoid macular edema groups (p < 0.001 and p < 0.001, respectively). However, there was no significant improvement after IVR injection in the SRD group (p = 0.252). In the SRD group, patients with ellipsoid zone disruption and external limiting membrane disruption demonstrated poorer visual gains at the last follow-up visit (p < 0.005 and p = 0.002, respectively).
Macular edema occurs in a wide variety of ocular diseases and is a common cause of vision loss in patients with diabetic retinopathy [1]. Its complex and multifactorial pathogenesis is not yet fully understood. What is apparent is that the multifactorial disruption of inner and outer blood-retinal barriers leads to an abnormal inflow of fluid into the neurosensory retina that exceeds the outflow, producing intraretinal and subretinal fluid accumulation [2345]. Using spectral domain optical coherence tomography (SD-OCT), Otani et al. [6] described three patterns of diabetic macular edema (DME): sponge-like swelling, cystoid macular edema (CME), and serous retinal detachment (SRD). SRD associated with CME can only be diagnosed using SD-OCT because it can be hidden beneath CME and therefore missed during fundus fluorescein angiography [7].
Vascular endothelial growth factor (VEGF) is a potent endothelial cell angiogenic factor and a powerful mediator of vascular permeability. It leads to the breakdown of the blood-retinal barrier in diabetic retinopathy, causing leakage of intravascular fluid from abnormal retinal capillaries and resulting in DME [8]. Therefore, treatment with anti-VEGF agents is one of the most promising approaches for the treatment of vision loss due to DME [910].
Various studies have established the safety and efficacy of anti-VEGF agents, including ranibizumab and bevacizumab, in the treatment of DME [111213]. However, only a few publications have addressed the issue of why some eyes respond to this treatment more readily than others. The presence of SRD in retinal vascular diseases, such as diabetes, may affect the treatment results for macular edema associated with retinal vascular leakage and may also limit the ability to perform effective macular laser treatment.
There are few reports about the results of bevacuzimab injection on different optical coherence tomographic patterns of DME [1415]. Koytak et al. [14] reported that the CME and SRD subtypes were associated with a greater reduction in central foveal thickness (CFT) than the diffuse retinal thickness (DRT) subtype. However, changes in visual acuity were not significantly different among the three groups.
The aim of this study was to evaluate the anatomical and functional outcomes based on various patterns of SD-OCT morphology in DME following treatment with intravitreal ranibizumab (IVR) injection.
A retrospective chart review of patients with DME who underwent SD-OCT evaluation in the department of ophthalmology between January 2011 and August 2014 was performed. This study adhered to the tenets of the Declaration of Helsinki and was approved by our local ethics committee (2015/01-21). Informed consent was obtained before the investigation began.
In this study, the medical records of patients who had received an IVR injection for the treatment of DME were retrospectively analyzed. Eyes that had clinically significant macular edema and a CFT of 300 µm or more determined by SD-OCT (Spectralis HRA+SD-OCT; Heidelberg Engineering, Heidelberg, Germany) were included in the analysis, regardless of diabetic retinopathy stage. If both eyes of the same patient met the inclusion criteria, only one eye was assigned randomly for the study.
All patients underwent macular SD-OCT measurements prior to IVR injection. Eyes that had poor quality SD-OCT scans were excluded from the study. The causes of these poor scans included media opacities, excessive blinking, or persistent eye movement. Other exclusion criteria were ocular surgery or trauma, intravitreal or periocular injection of any drug, or laser photocoagulation within six months of the injection; history of any previous vitreoretinal surgical procedure; presence of concomitant retinal pathologies and glaucoma or evidence of vitreomacular traction on SD-OCT. Patients with a follow-up time shorter than six months were also excluded from the study.
During SD-OCT examination, the macula was scanned in six radial sections, including the horizontal, vertical, and oblique planes, through the center of the fovea. The macular thickness was measured automatically by the topography software built into the SD-OCT device.
The IVR injections were performed in the operating room under aseptic conditions. Topical anesthesia was achieved by instillation of at least three drops of 0.5% proparacaine hydrochloride (Alcaine; Alcon Laboratories, Fort Worth, TX, USA). Povidone iodine (5%) was applied to the lids and eyelashes and instilled in the conjunctiva before draping. Next, 0.5 mg/0.05 mL of ranibizumab (Lucentis; Novartis Pharma AG, Basel, Switzerland) was injected using a 30-gauge needle 4 mm posterior to the limbus (3.5 mm in pseudophakic eyes). Finally, a drop of 5% povidone iodine was instilled at the injection site. An eye pad was placed, and 0.5% moxitifloxacin topical drops were prescribed for instillation four times daily.
Best-corrected visual acuity (BCVA) with a Snellen chart (BCVA measurements were converted to logarithm of the minimum angle of resolution [logMAR]) and CFT values assessed with SD-OCT prior to the IVR injection and at the last visit were recorded. Eyes were divided into SRD, DRT, and CME groups according to the assessment of macular edema morphology on SD-OCT (Fig. 1A–1C). When more than one edema pattern was observed, the eye was included in the group of the most obviously predominant pattern. In cases where more than one pattern was present and none was obviously predominant, the eye was not included in the study. The BCVA, macular appearance and SD-OCT findings were used to determine whether the patient should receive a repeat injection of IVR. However, it was left at the discretion of the treating physician to follow a specific regimen or treat patients on an as-needed basis.
All data were analyzed using the SPSS ver. 11.5 (SPSS Inc., Chicago, IL, USA). BCVA measurements were converted to logMAR equivalents for statistical analysis. Pearson chi-square test was used for comparative analyses of categorical variables. The independent sample t-test and paired t-test were employed to analyze changes in BCVA and CFT. One-way analysis of variance (post-hoc Tukey) was used to assess variations among the three groups. For all statistical tests, p < 0.05 was considered significant.
One hundred thirty-four eyes of 134 patients with a minimum follow-up period of six months were included in the study. The mean follow-up time was 9.4 ± 3.4 months (range, 6 to 24 months). Of the 134 patients, 69 (51.5%) were female, and 65 (48.5%) were male. The mean age was 61.4 ± 9.2 years (range, 44 to 81 years). The baseline demographic and clinical properties of the patients in each group are summarized in Table 1. The three groups did not differ significantly in terms of age and gender (p = 0.266 and p = 0.822 respectively). Table 2 and 3 summarize the BCVA (logMAR) and SD-OCT measurement data before and after the injection. Pre-injection mean BCVA did not differ significantly between groups (p = 0.063). The difference between mean pre-injection CFT values of the three groups was also not statistically significant (p = 0.362). When the pre-injection and post-injection data were compared within each group, increases in the BCVA were statistically significant in the DRT and CME groups (p < 0.001 and p < 0.001, respectively), but was not significant in the SRD group (p = 0.252). However, the mean CFT values significantly decreased in all three groups (p < 0.001). There was also a statistically significant difference between groups in terms of postoperative BCVA (p < 0.001). The three groups showed no significant variation in post-injection CFT (p = 0.825). In the SRD group, 34.8% (16 / 46) had visual improvement; 45.7% (21 / 46) had the same BCVA at the last visit as at preinjection; and 19.6% (9 / 46) of the patients demonstrated deterioration of BCVA at the last visit compared with the pre-injection BCVA. In the DRT group, 80% (40 / 50) experienced visual improvement; the BCVA was the same as the pre-injection BCVA at the last visit in 8.0% (4 / 50); and 12.0% (6 / 50) showed deterioration of the BCVA at the last visit compared with pre-injection BCVA. In the CME group, 78.9% (30 / 38) displayed visual improvement; 13.2% (5 / 38) had no change in BCVA at the last visit; and 7.9% (3 / 38) experienced deterioration in BCVA at the last visit compared with pre-injection BCVA.A decrease >50 µm in the CFT was accepted as a decreased CFT, while any change ≤50 µm was accepted as no change in CFT. An increase >50 µm was defined as an increased CFT. In the SRD group, 71.7% (33 / 46) had a decreased CFT, while 80% (40 / 50) in the DRT group and 81.6% (31 / 38) in the CME group experienced a decreased CFT. Fig. 2 shows the results of all three groups with regard to the change in CFT. We also evaluated the improvement rates of BCVA in eyes with a decreased CFT. In the SRD group, 39.4% of eyes with a decreased CFT also demonstrated improvement in the BCVA; these rates were 82.9% and 74.2% in the DRT and CME groups, respectively (Fig. 3).
Shifts in the pathomorphology of the macular edema were assessed in each subgroup. In the DRT group, 48.0% (24 / 50) of the eyes became dry with no or minimal fluid, and 16.0% (8 / 50) retained DRT morphology; 28% became CME (14 / 50), while only 8% (4 / 50) were found to have SRT. In the CME group, 63.2% (24 / 38) of the eyes developed dryness, 36.8% (14 / 38) continued to have CME, and no patient was found to have SRD or DRT. For the SRD group, 21.7% (10 / 46) of the eyes became dry, 19.6% (9 / 46) and 17.4% (8 / 46) of the eyes converted to having DRT and CME, respectively, while 41.3% (19 / 46) remained with SRD.
An analysis of the outer retinal structures (including the external limiting membrane [ELM] and inner segment ellipsoidal band layer [previously known as the boundary of the inner segment and outer segment junction]) was carried out; ELM was found to be disrupted in 12 eyes (26%) in the SRD group, 6 eyes (12%) in the DRT group, and 6 eyes (15.8%) in the CME group. The ellipsoidal layer was disrupted in 10 eyes (21.7%) in the SRD group, 7 eyes (14%) in the DRT group, and 5 eyes (13.2%) in the CME group. In the DRT and CME groups, patients with an intact ELM and integrity of the ellipsoid zone layer showed significant visual improvement post-injection compared with eyes with disruptions of the ELM and the ellipsoid zone layer (p = 0.004 and p = 0.008, respectively). However, in the SRD group, patients with ellipsoid zone and ELM disruption experienced poorer visual gains at the last follow-up visit (p < 0.005 and p = 0.002, respectively).
No inflammation or severe decreases in vision immediately following the injection were noted, although 7 (5.2%) eyes (3 in the SRD group, 2 in the DRT group, and 2 in the CME group) showed elevated IOP. These cases were managed with conservative medical treatment. At the final follow- up, no ocular or systemic adverse events, such as thromboembolic events (cerebrovascular accidents, transient ischemic attacks, myocardial infarctions, or peripheral vascular diseases), were reported.
The main cause of vision impairment in diabetic patients is DME. A large epidemiological study indicated that macular edema was present in 15% of patients with diabetic retinopathy [16]. The pathogenesis of DME is complex and multifactorial. Disruption of the inner and outer blood-retinal barriers leads to abnormal inflow of fluid into the neurosensory retina that exceeds the outflow, producing intraretinal and subretinal fluid accumulation [23]. However, the specific details of the pathogenesis of DME remain unclear. Classification of DME according to the SD-OCT findings might be helpful to obtain more information about the pathogenesis of DME. The first SD-OCT classification of DME was reported by Otani et al. [6]. Although various patterns of DME have been recognized on SD-OCT, few published studies have reported the visual outcomes for these SD-OCT patterns in DME patients treated with anti-VEGF therapy. Kim et al. [17] concluded that the intravitreal injection of bevacizumab was more effective in the DRT type than in the CME or SRD types of DME. Wu et al. [18] found that patients with cystoid changes gained greater improvements in visual acuity and macular thickness and volume after intravitreal bevacizumab injection. However, Koytak et al. [14] stated that there was no statistically significant variation between focal, cystoid, and neurosensory detachment groups regarding changes in BCVA after injection of intravitreal bevacizumab.
Shimura et al. [19] reported that the foveal thickness in all patterns was reduced, but the reduction ratios in the DRT and CME groups were significantly greater than that seen in the SRD group following intravitreal bevacuzimab injection. Similarly, improvement in visual acuity in the DRT and CME groups was significantly greater than that in the SRD group; therefore, it was concluded that the effectiveness of intravitreal bevacuzimab injection in reducing macular edema varied depending on the SD-OCT pattern and was greatest in the DRT group, intermediate in the CME group, and weakest in the group with the SRD pattern. In our study, the pre-injection BCVA and CFT values were similar in all three groups. The observed decrease in CFT at the last follow-up visit was not statistically significant between the three groups, whereas the improvement in BCVA was statistically worse in the SRD group compared with the DRT and CME groups. The ELM and ellipsoid zone integrities were significantly correlated with post-treatment visual acuity and were significantly lower in the SRD type than in the other types. In eyes with SRD, outer retinal structures were found to be associated with poor visual recovery. In our series, 34 of 41 DRT eyes (82.3%) with a decrease >50 µm also showed improvement in BCVA. In the CME group, 23 of 31 (74.2%) eyes with a decrease >50 µm demonstrated improvement in BCVA. However, in the SRD group, only 13 of 33 (39.4%) eyes with a decrease >50 µm experienced an improvement in BCVA. In 2016, Seo et al. [20] reported that vision gains and retinal anatomy improvement were maintained in all three types during the first year of IVR administration, which is in agreement with our study findings. Additionally, the BCVA of SRD (20 / 60) patients was significantly worse than that of the other types (DRT = 20 / 38; CME = 20 / 43) after 12 months. In contrast, Giocanti-Auregan et al. [21] found in 2017 that similar BCVA gains were observed regardless of the presence of SRD. The higher visual gain usually observed in DME with SRD could be associated with a lower baseline BCVA.
The pathogenesis of DRT involves the persistent breakdown of the inner blood retinal barrier from the loss of anchor proteins in the tight junctions of the capillary endothelial cells [22]. Vascular endothelial growth factor is a pluripotent growth factor that acts as an endothelial cell-specific mitogen and vasopermeability factor and thus plays a critical role in promoting angiogenesis and vascular leakage [232425]. Therefore, IVR reduces hypervasopermeability, leading to a reduction of DRT. Although the pathogenesis of CME remains unknown, the reduction of CME seen with intravitreal bevacizumab and IVR injections suggests that CME formation is partly dependent on VEGF. The pathogenesis of SRD associated with DME occurs due to transient migration of fluid from the cystoid spaces in the retina to the subretinal space [7]. Another theory is that SRD occurs subsequent to failure of the retinal pigment epithelium (RPE) pump mechanism [26]. The breakdown of the RPE pump or disruption of the tight junctions between adjacent RPE cells results in intraretinal edema and SRD [272829]. IVR may have little efficacy in the regression of diabetes-induced RPE impairment, which suggests that VEGF is not primarily responsible for SRD formation in DME.
In conclusion, the visual improvement noted in DME eyes with SRD was lower than that experienced by eyes with DRT and CME. Disruption of the photoreceptor integrity was correlated with a poorer visual outcome and appeared more frequently in the SRD group. Based on our findings, we believe that different treatment modalities should be considered for DME with SRD. More prospective studies are warranted to better understand the pathogenesis of DME.
Figures and Tables
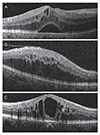 | Fig. 1Diabetic macular edema (DME) classification based on spectral domain optical coherence tomography. (A) Patients with predominant serous retinal detachment DME had an associated subretinal collection of fluid under the fovea. (B) Diffuse DME patients demonstrated widespread retinal thickening with a sponge-like appearance of the macula. (C) Patients with focal cystoid DME had a mound-like appearance of the fovea due to focal collection of fluid at the fovea. |
 | Fig. 2Central foveal thickness (CFT) changes of the groups. SRD = serous retinal detachment; DRT = diffuse retinal thickness; CME = cystoid macular edema. |
 | Fig. 3Results of best-corrected visual acuity (BCVA) in eyes with decreased diabetic macular edema. SRD = serous retinal detachment; DRT = diffuse retinal thickness; CME = cystoid macular edema. |
References
1. Patz A, Schatz H, Berkow JW, et al. Macular edema: an overlooked complication of diabetic retinopathy. Trans Am Acad Ophthalmol Otolaryngol. 1973; 77:OP34–OP42.
2. Do Carmo A, Ramos P, Reis A, et al. Breakdown of the inner and outer blood retinal barrier in streptozotocin-induced diabetes. Exp Eye Res. 1998; 67:569–575.

3. Sander B, Larsen M, Moldow B, Lund-Andersen H. Diabetic macular edema: passive and active transport of fluorescein through the blood-retina barrier. Invest Ophthalmol Vis Sci. 2001; 42:433–438.
4. Tso MO, Cunha-Vaz JG, Shih CY, Jones CW. Clinicopathologic study of blood-retinal barrier in experimental diabetes mellitus. Arch Ophthalmol. 1980; 98:2032–2040.

5. Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol. 2009; 54:1–32.

6. Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999; 127:688–693.

7. Kang SW, Park CY, Ham DI. The correlation between fluorescein angiographic and optical coherence tomographic features in clinically significant diabetic macular edema. Am J Ophthalmol. 2004; 137:313–322.

8. Ciulla TA, Harris A, Latkany P, et al. Ocular perfusion abnormalities in diabetes. Acta Ophthalmol Scand. 2002; 80:468–477.

9. Thomas BJ, Shienbaum G, Boyer DS, Flynn HW Jr. Evolving strategies in the management of diabetic macular edema: clinical trials and current management. Can J Ophthalmol. 2013; 48:22–30.

10. Stewart MW. Anti-vascular endothelial growth factor drug treatment of diabetic macular edema: the evolution continues. Curr Diabetes Rev. 2012; 8:237–246.
11. Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010; 33:2399–2405.

12. Diabetic Retinopathy Clinical Research Network. Scott IU, Edwards AR, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007; 114:1860–1867.

13. Arevalo JF, Sanchez JG, Wu L, et al. Primary intravitreal bevacizumab for diffuse diabetic macular edema: the Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology. 2009; 116:1488–1497.
14. Koytak A, Altinisik M, Sogutlu Sari E, et al. Effect of a single intravitreal bevacizumab injection on different optical coherence tomographic patterns of diabetic macular oedema. Eye (Lond). 2013; 27:716–721.

15. Cheema HR, Al Habash A, Al-Askar E. Improvement of visual acuity based on optical coherence tomography patterns following intravitreal bevacizumab treatment in patients with diabetic macular edema. Int J Ophthalmol. 2014; 7:251–255.
16. Petrella RJ, Blouin J, Davies B, Barbeau M. Prevalence, demographics, and treatment characteristics of visual impairment due to diabetic macular edema in a representative Canadian cohort. J Ophthalmol. 2012; 2012:159167.

17. Kim M, Lee P, Kim Y, et al. Effect of intravitreal bevacizumab based on optical coherence tomography patterns of diabetic macular edema. Ophthalmologica. 2011; 226:138–144.

18. Wu PC, Lai CH, Chen CL, Kuo CN. Optical coherence tomographic patterns in diabetic macula edema can predict the effects of intravitreal bevacizumab injection as primary treatment. J Ocul Pharmacol Ther. 2012; 28:59–64.

19. Shimura M, Yasuda K, Yasuda M, Nakazawa T. Visual outcome after intravitreal bevacizumab depends on the optical coherence tomographic patterns of patients with diffuse diabetic macular edema. Retina. 2013; 33:740–747.

20. Seo KH, Yu SY, Kim M, Kwak HW. Visual and morphologic outcomes of intravitreal ranibizumab for diabetic macular edema based on optical coherence tomography patterns. Retina. 2016; 36:588–595.

21. Giocanti-Auregan A, Hrarat L, Qu LM, et al. Functional and anatomical outcomes in patients with serous retinal detachment in diabetic macular edema treated with ranibizumab. Invest Ophthalmol Vis Sci. 2017; 58:797–800.
22. Antonetti DA, Barber AJ, Khin S, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998; 47:1953–1959.

23. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331:1480–1487.

24. Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999; 13:9–22.

25. Grant MB, Afzal A, Spoerri P, et al. The role of growth factors in the pathogenesis of diabetic retinopathy. Expert Opin Investig Drugs. 2004; 13:1275–1293.

26. Gaucher D, Sebah C, Erginay A, et al. Optical coherence tomography features during the evolution of serous retinal detachment in patients with diabetic macular edema. Am J Ophthalmol. 2008; 145:289–296.

27. Ozdemir H, Karacorlu M, Karacorlu S. Serous macular detachment in diabetic cystoid macular oedema. Acta Ophthalmol Scand. 2005; 83:63–66.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download