Abstract
Inspissated bile syndrome (IBS) is a relatively rare condition. Many treatment options are available, including medication, surgery, and surgical interventions, such as insertion of cholecystostomy drain, endoscopic retrograde cholangiopancreatography, internal biliary drainage, and percutaneous transhepatic biliary drainage (PTBD). We herein report the first case of IBS that was successfully treated with PTBD in a two-month-old infant in Korea. PTBD was initiated on postnatal day 72. On postnatal day 105, we confirmed complete improvement and successfully removed the catheters. This report suggests that PTBD is a viable and safe treatment option for obstructive jaundice in very young infants.
Inspissated bile syndrome (IBS) is defined as the obstruction of the extrahepatic duct by a bile plug, sludge without bile duct malformation, congenital chemical defects of the bile, or hepatocellular lesions.1 This inspissation of bile and mucus within the bile ducts is usually caused by blood transfusion, prolonged parenteral nutrition, or diuretics.23 Management options include medication, surgery, chemotherapy, radiotherapy, and invasive surgical interventions, such as a percutaneous cholecystostomy, endoscopic retrograde cholangiopancreatography, internal biliary drainage, and percutaneous transhepatic biliary drainage (PTBD).4567 However, there are limited surgical and procedural options for newborns, because these treatments are invasive, difficult, and frequently accompanied by postoperative complications. Consequently, very few reports of such cases are present in the literature. We herein describe our experience with a two-month-old infant presenting with IBS who successfully underwent PTBD.
A female newborn weighing 2790 g at 36+6 weeks of gestation was born at a local hospital through vaginal delivery with meconium staining. The Apgar score was 5 at both 1 and 5 min. The baby displayed no movement or respiratory drive. Thus, positive pressure ventilation was performed, and she was then transferred to the newborn intensive care unit.
The newborn had features of meconium aspiration, hypoxic ischemic encephalopathy, disseminated intravascular coagulation, and retinal hemorrhage. Gastrointestinal motility was decreased, and there was intermittent gastrointestinal bleeding for 20 days. Initial feeding was started on postnatal day one; however, continuation of feeding was difficult due to recurrent ileus and bleeding. An initial abdominal ultrasonography conducted on the second day after birth showed normal findings, but at 40 days of age, abdominal ultrasonography detected sludge in the gall bladder. Cycles of improvement and deterioration of the sludge in the gall bladder continued thereafter. Total parenteral nutrition (TPN) was started on the first day, and 100 mL/kg of enteral feeding was achieved on the 57 days of age. TPN was discontinued at 66 days of age.
Fifty-five days after birth, liver function tests showed abnormal findings with elevation of aspartate aminotransferase (AST) 1086 IU/L, alanine aminotransferase (ALT) 679 IU/L, and γ-glutamyl transpeptidase 399 IU/L (Fig. 1). Coagulation times were in the normal range. Congenital TORCH infection was excluded. Ursodeoxycholic acid (UDCA) was started for treatment of hepatitis and was continued for 18 days. Multivitamins and phenobarbital were administered together. At 71 days of age, acholic stools were noted, and the infant was confirmed as having cholestatic jaundice (total bilirubin 14.0 mg/dL, RR <1.2 mg/dL; direct bilirubin 7.8 mg/dL, RR <0.2 mg/dL). Ultrasonography revealed the presence of newly developed common bile duct sludge (length approximately 2.0 cm) and extra- and intra-hepatic bile duct dilatation (Fig. 2A).
PTBD was performed under ultrasound guidance in our interventional radiology suite at 72 days postnatally. The patient was intubated, and vital signs were monitored during the procedure. Pain was managed with fentanyl citrate. A 21-G needle was advanced under ultrasonographic guidance, using color Doppler to avoid vessels with a sub-xiphoid approach. After puncturing the intrahepatic duct branch B2, contrast material was injected, and correct positioning was confirmed by opacification of the intrahepatic duct. A 0.018-guidewire was passed into the common bile duct, and a 5-Fr Pigtail catheter (Cook Endoscopy Inc., Limerick, Ireland) was placed there (Fig. 3).
After PTBD insertion, natural drainage of approximately 15–114 mL of greenish bile was done daily. Patient follow-up was done via clinical assessment, liver function tests, bilirubin level tests, and abdominal ultrasonography (Fig. 2B). Four weeks after PTBD initiation, a follow-up cholangiogram was performed to confirm resolution of dilatation of the biliary tree and complete removal of the bile plug. Subsequently, the pigtail catheter was removed without complications.
IBS, first described in 1935, is diagnosed based on medical history and typical ultrasound features.8 The incidence in England is 1 in 175000 live births, accounting for about 8% of all cases of surgical jaundice during infancy.2
Infants with biliary obstruction develop jaundice, and pass pale stool and dark urine.9 Blood tests reveal elevated AST, ALT, and direct bilirubin levels. The pathological findings are nonspecific.1 In present case, elevated liver enzymes and serum bilirubin levels, as well as clinical symptoms, were consistent with the findings of previous studies. The causes of biliary obstruction without structural abnormality include long-term fasting, long-term TPN, hemolytic anemia, hepatitis, transfusion, and antibiotics, such as ceftriaxone or diflucan.89101112 In our case, long-term fasting, transfusion, and TPN could have caused IBS. Treatment of IBS includes various challenges. In some cases, biliary sludge reportedly resolved spontaneously without treatment within 1 week.1314 In one case, omega-3 PUFAs were used as an alternative to surgery.15 In some medical trials, use of UDCA alone to dissolve the bile sludge failed to improve biochemical test results, and ultimately, surgery was required.716 Surgical therapy is normally only needed when the extrahepatic bile ducts are dilated to more than 3 mm. Among the various surgical interventions, percutaneous transhepatic cholangiography is difficult in infants because of the small size of the intrahepatic gall ducts.6 Failure of this treatment will entail a laparotomy for drainage of the inspissated bile.
The feasibility of PTBD in adults and children has been demonstrated, such as for the palliative treatment of inoperable cases, decompression before surgery, recurrent obstructive jaundice after surgery, purulent cholangitis and hepatic abscess, and biliary interventions for gallstones and bile duct biopsy. In contrast to adult cases, temporary biliary drainage could be used in children without congenital anomalies.17 However, published data concerning its usage in infants are very limited. A study on PTBD for obstructive jaundice in a three-month-old child with a brain tumor was reported recently.18 Therefore, in newborns or infants without congenital anomalies, PTBD should be considered if recovery does not occur after active feeding and medical treatment in order to prevent invasive surgery and progression to hepatic failure.
To our knowledge, this is the first report from Korea to describes a case of obstructive jaundice treated by PTBD in a two-month-old infant. PTBD appears to be a safe and effective treatment for obstructive jaundice in very young infants.
Figures and Tables
 | Fig. 1Results from liver function and serum bilirubin tests. (A) The liver function test during the patient's clinical course showed gradual improvement after PTBD. (B) Serum bilirubin level during the infant's clinical course showed marked improvement after PTBD. *Medication start, †PTBD insertion, ‡PTBD removal. AST, aminotransferase; ALT, alanine aminotransferase; PTBD, percutaneous transhepatic biliary drainage. |
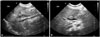 | Fig. 2Abdominal ultrasound imaging before and after PTBD. (A) On the day before PTBD, a 2.0 cm sized common bile duct (CBD) sludge and multiple bile sludges at not-tensile gall bladder, as well as extrahepatic/intrahepatic bile duct dilatation, were observed. (B) Three days after PTBD, extrahepatic/intrahepatic bile duct dilatation had improved. PTBD, percutaneous transhepatic biliary drainage. |
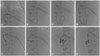 | Fig. 3Radiographic findings of percutaneous transhepatic biliary drainage procedures. The intrahepatic duct branch was punctured with a 21-G needle (A–C), and the guidewire was passed into the common bile duct (D–F). The needle was replaced with a 5-Fr pigtail catheter and percutaneous transhepatic biliary drainage was achieved (G and H). There is a plug in the distal common bile duct, visible as a filling defect. |
References
1. Bernstein J, Braylan R, Brough AJ. Bile-plug syndrome: a correct-able cause of obstructive jaundice in infants. Pediatrics. 1969; 43:273–276.

2. Davenport M, Betalli P, D'Antiga L, Cheeseman P, Mieli-Vergani G, Howard ER. The spectrum of surgical jaundice in infancy. J Pediatr Surg. 2003; 38:1471–1479.

3. Lightwood R, Bodian M. Biliary obstruction associated with icterus gravis neonatorum. Arch Dis Child. 1946; 21:209–217.

4. Bollu BK, Dawrant MJ, Thacker K, Thomas G, Chenapragadda M, Gaskin K, et al. Inspissated bile syndrome; safe and effective minimally invasive treatment with percutaneous cholecystostomy in neonates and infants. J Pediatr Surg. 2016; 51:2119–2122.

5. Helin R, Bhat R, Rao B. Ultrasound-guided percutaneous cholecystostomy for acute neonatal biliary obstruction. Neonatology. 2007; 91:266–270.

6. Duman L, Büyükyavuz BI, Akcam M, Koroglu M, Tepeli H. Percutaneous management of bile-plug syndrome: a case report. J Pediatr Surg. 2011; 46:e37–e41.

7. Gunnarsdóttir A, Holmqvist P, Arnbjörnsson E, Kullendorff CM. Laparoscopic aided cholecystostomy as a treatment of inspissated bile syndrome. J Pediatr Surg. 2008; 43:e33–e35.

8. Brownschidle S, Sullivan J, Sartorelli K, Potenta S, Zenali M. Neonatal cholestasis due to biliary sludge-review and report of a case associated with use of diflucan. Ann Clin Path. 2014; 2:1018.
9. Fawaz R, Baumann U, Ekong U, Fischler B, Hadzic N, Mack CL, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2017; 64:154–168.

11. Doty JE, Pitt HA, Porter-Fink V, DenBesten L. The effect of intravenous fat and total parenteral nutrition on biliary physiology. JPEN J Parenter Enteral Nutr. 1984; 8:263–268.

12. Soysal A, Eras¸ov K, Akpinar I, Bakir M. Biliary precipitation during ceftriaxone therapy: frequency and risk factors. Turk J Pediatr. 2007; 49:404–407.
13. Lang EV, Pinckney LE. Spontaneous resolution of bile-plug syndrome. AJR Am J Roentgenol. 1991; 156:1225–1226.

14. Jee KB, Song JY, You KY, Min KS, Kim DH, Lee KS. A case of spontaneous resolution of bile plug syndrome in a 4-year-old girl. Korean J Pediatr Gastroenterol Nutr. 1999; 2:262–266.

15. Jun WY, Cho MJ, Han HS, Bae SH. Use of omega-3 polyunsaturated fatty acids to treat inspissated bile syndrome: a case report. Pediatr Gastroenterol Hepatol Nutr. 2016; 19:286–290.

16. Berger S, Schibli S, Stranzinger E, Cholewa D. One-stage laparoscopic surgery for inspissated bile syndrome: case report and review of surgical techniques. Springerplus. 2013; 2:648.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download