Abstract
Objective
To compare the diagnostic performance of electrocardiogram (ECG)-gated thoracic computed tomography angiography (TCTA) without heart rate (HR) control in ischemic stroke patients with coronary CTA (CCTA) in non-stroke patients for detection of significant coronary artery stenosis.
Materials and Methods
From September 2009 through August 2014, we retrospectively enrolled 138 consecutive patients diagnosed with acute ischemic stroke who had undergone ECG-gated TCTA and conventional coronary angiography (CCA). Over the same period, we selected 167 non-stroke patients with suspected or known coronary artery disease who had undergone CCTA and CCA. With CCA as the reference standard, the diagnostic performance of TCTA and CCTA for identification of significant coronary stenosis (diameter reduction ≥ 50%) was calculated.
Results
There was no significant difference in baseline characteristics between TCTA (n = 132) and CCTA (n = 164), except for the higher prevalence of atrial fibrillation in the stroke group. There was significant difference (p < 0.001) between TCTA and CCTA in average HR (68 ± 12 vs. 61 ± 10 beats per minute) and image quality score (1.3 ± 0.6 vs. 1.2 ± 0.6). Significant coronary stenosis was identified in 101 (77%) patients, 179 (45%) vessels, and 293 (15%) segments of stroke patients, and in 136 (83%) patients, 259 (53%) vessels, and 404 (16%) segments of non-stroke patients. Diagnostic performance on a per-vessel and per-patient basis was similar in both TCTA and CCTA groups. There was only significant difference in area under receiver-operating characteristic curve between TCTA and CCTA groups (0.79 vs. 0.87, p < 0.001) on per-segment basis.
Stroke is one of the leading causes of death and disability worldwide (1). Ischemic strokes constitute an estimated 70–80% of all strokes (2). Embolism of cardiac origin accounts for around 15–30% of ischemic stroke. It results from primary intracardiac embolic sources, intracardiac shunts, and pulmonary arteriovenous malformations serving as conduits for paradoxical embolism, and atherosclerotic plaques of the ascending thoracic aorta and aortic arch (3456). For the last decade, coronary computed tomography angiography (CCTA) has been as effective as transesophageal echocardiography (TEE) in detecting cardioembolic sources in patients with ischemic stroke (78).
Ischemic stroke and coronary artery disease (CAD) frequently coexist. They share common risk factors. Prevalence of significant coronary stenosis (luminal diameter reduction of ≥ 50%) is 18–38% in patients with ischemic stroke or transient ischemic attack (91011). In addition, CAD is the major cause of death during follow-up of patients with ischemic stroke (1213). Therefore, detection and treatment of concomitant CAD is important for improved long-term prognosis and survival in ischemic stroke patients (14).
Coronary computed tomography angiography has been widely used for the accurate diagnosis of CAD (151617). However, CCTA only provides anatomic information of the heart and the proximal portion of the ascending thoracic aorta in patients with ischemic stroke (78). Electrocardiogram (ECG)-gated thoracic CTA (TCTA) may be useful for the assessment of cardiothoracic diseases with high-resolution images of the heart, aorta, and lungs and facilitates the evaluation and diagnosis of cardioembolic sources of embolism in patients with ischemic stroke (18).
Until now, no study has addressed the potential added value of TCTA for detection of CAD concurrently in ischemic stroke patients. If the diagnostic accuracy of the TCTA without heart rate (HR) control for detection of concomitant CAD is comparable to CCTA, then TCTA could potentially facilitate a comprehensive assessment of the cardioembolic source and CAD in ischemic stroke patients in a single CT scan. Such an approach reduces unnecessary diagnostic procedures, overall exposure to radiation, and length of hospitalization. The aim of this study was to compare the diagnostic performance of dedicated TCTA without HR control in ischemic stroke patients with that of dedicated CCTA in non-stroke patients to detect significant coronary artery stenosis.
This study was approved by the hospital Institutional Review Board (Konkuk university medical center, Seoul, Korea) with informed consent waived. Patients with acute ischemic stroke who were admitted to the Neurology Department of Konkuk university medical center between September 2009 and August 2014 were retrospectively enrolled. One hundred thirty-eight patients met the following inclusion criteria: evaluation with brain CT and/or brain magnetic resonance imaging to confirm acute ischemic stroke, evaluation with ECG-gated TCTA for cardiogenic or aortogenic sources of embolism, and evaluation with conventional coronary angiography (CCA) within one month after TCTA.
Over the same period, 470 non-stroke patients with suspected or known CAD underwent CCTA and CCA within one month. A total of 167 matched controls (age, sex, and coronary risk factors) were selected to compare the diagnostic performance of TCTA versus CCTA in the evaluation of significant coronary stenosis.
Among the 138 stroke patients, 6 (4.3%) were excluded because of poor image quality related to severe cardiac motion artifact (n = 4) and respiratory motion artifact (n = 2). Among 167 non-stroke patients with suspected or known CAD, 3 (1.8%) were excluded because of non-diagnostic image quality related to severe cardiac motion artifact (n = 2) and respiratory motion artifact (n = 1). Overall, the study population consisted of 132 stroke patients and 164 non-stroke patients.
All CT examinations were performed using a first-generation dual-source CT system (Somatom Definition; Siemens Medical Solutions, Forchheim, Germany) with the following scan parameters: collimation, 64 × 0.6 mm; slice acquisition, 64 × 0.6 mm, using the z-flying focal spot technique; gantry rotation time, 330 ms; pitch 0.20–0.43 adapted to the HR; tube voltage, 100 kV; and various mAs per rotation tube current-time product according to study protocol (90–120 mAs for early-phase TCTA, 240–340 mAs for late-phase TCTA, and 100–280 mAs for CCTA). For coronary calcium scoring, we performed the non-enhanced, prospective ECG gating coronary CT with 75% R-R interval. A retrospective ECG-gating, helical CT was used for CCTA. To reduce the radiation dose, we used the different CT acquisition protocol depending on the patients' HR and variability. The ECG-based, tube current dose modulation was used in patients with HR < 90 bpm. In particular, the full tube current of 65–75% of R-R interval was used in patients with stable HR < 65 bpm and the full tube current of 20–70% of R-R interval was used in patients with a stable HR between 65–89 bpm. ECG-based tube current modulation was switched off in patients with a HR ≥ 90 bpm or with an irregular cardiac rhythm.
Iopromide (Ultravist 370®; Bayer Healthcare, Berlin, Germany) was used for the injection of intravenous contrast using a dual-head power injector (Stellant D; Medrad, Indianola, PA, USA). Contrast agent application was controlled by a bolus tracking technique. For TCTA studies, contrast material was administered at a rate of 4.0 mL/s and a two-phase injection protocol. First, 85–100 mL iopromide was administered followed by 40 mL of saline. For CCTA studies, a three-phase bolus was used at a rate of 4.5 mL/s. First, 65–80 mL of undiluted contrast media was administered followed by 45 mL of a mixture of 70% contrast and 30% saline with a saline chaser.
All patients received 0.6 mg nitroglycerin sublingually immediately prior to the non-enhanced ECG-gated CT scan, prospectively triggered at 75% of the R-R interval for the measurement of the coronary calcium score. TCTA was performed retrospectively with ECG-gated scan without HR control using a beta-blocker, and with a wide field of view from the aortic arch to the inferior border of the heart. Three minutes after the end of the early-phase acquisition, a late-phase scan was conducted to differentiate between slow flow or spontaneous echo contrast and thrombus in left atrial appendage (LAA) using the prospective ECG triggering technique (75% of the R-R interval) and CARE DOSE 4D (Siemens Medical Solutions) encompassing the left atrium to the middle of the left ventricle to reduce radiation exposure (Fig. 1). For the CCTA, patients with a pre-scan HR > 65 bpm were administered 50–100 mg metoprolol orally 1 hour prior to CCTA. Retrospective ECG-gated CCTA was performed from 1 cm below the level of the tracheal bifurcation to the inferior border of the heart in a craniocaudal direction.
Early-phase TCTA and CCTA images were previewed automatically. The optimal mid-diastolic and end-systolic phases were selected to obtain appropriate reconstruction time points using reconstruction software (BestDiast/BestSyst®; Siemens Medical Solutions). CT images were reconstructed with a slice thickness of 0.75 mm, reconstruction increment of 0.4 mm, and a medium soft tissue convolution kernel (B26f). Depending on the individual anatomy, the reconstructed field-of-view was adjusted to precisely encompass the heart (image matrix 512 × 512 pixels). CT data sets were transferred to an external workstation (Vitrea 2; Vital Images, Plymouth, MN, USA) and reviewed via multiplanar reformations.
Thoracic computed tomography angiography and CCTA images were assessed by two independent radiologists with 14 years and 5 years of experience, respectively, associated with the presence and degree of stenosis in each coronary segment and vessel. Both radiologists were blinded to the clinical data and the results of CCA imaging tests. Disagreements between readers were resolved by consensus. For qualitative assessment, a single radiologist also rated the overall image quality of CT on a scale of 1–4, with 1 being excellent, 2 being good, 3 being adequate, and 4 being poor/non-diagnostic (19).
The coronary arteries were evaluated according to a 3-vessel and 16-segment coronary artery model modified from the American Heart Association classification (20). Each segment was classified as either non-significant (< 50% reduction in lumen diameter) or significant (≥ 50% reduction in lumen diameter) stenosis. Non-diagnostic segments were not evaluated because of cardiac or respiratory motion artifacts, severe calcification or stenting. We considered a non-diagnostic coronary segment as significant stenosis to avoid the risk of missing stenoses potentially present in non-diagnostic segments.
The findings in LAA from two-phase TCTA were classified into three categories: no filling defect in both the early- and late-phase images; an early filling defect, which was seen in LAA in only the early-phase images, and was absent in the late-phase images; and a persistent filling defect apparent in both early- and late-phase images. In TCTA, an early filling defect was considered an early filling artefact or spontaneous echo contrast whereas a persistent filling defect was considered a thrombus (21). Patent foramen ovale was defined as a channel-like communication of the interatrial septum with the presence of a contrast material jet from the left atrium to the right atrium perpendicular to the septum. Interatrial septal aneurysm was diagnosed by the enlargement of the interatrial septal membrane more than 10–15 mm into either the left or the right atrium. Hypokinetic segmental myocardial wall was defined as reduced movement or wall thickening of a segment of the heart muscle. Presence or absence of atherosclerotic plaque was assessed in the ascending aorta and aortic arch (18).
Quantitative assessment of stenosis severity on CCA was performed using commercially available software in accordance with societal recommendations (CAAS; Pie Medical, Maastricht, The Netherlands). CCAs were interpreted by an experienced interventional cardiologist with 30 years of experience. Similar to CCTA, a vessel was diagnosed with significant stenosis if maximal vessel diameter reduction by CCA was ≥ 50% in any coronary segment in any angiographic view.
An effective radiation dose was calculated for all patients. The dose-length product (DLP, measured in milligray-centimeters) was defined as the volume CT dose index multiplied by scan length. DLP was an indicator of the integrated radiation dose of the entire CT examination. It was displayed on the dose report from the CT scanner and was recorded. A reasonable approximation of the effective CT radiation dose was calculated by multiplying DLP with a conversion coefficient for the chest (κ = 0.014 mSv·mGy−1· cm−1) (22).
The categorical data are presented as frequencies and percentages. The data were compared using the χ2-test. The normally distributed continuous data are presented as means ± standard deviations. They were compared using the two-tailed t test for independent samples. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated from 2 × 2 contingency tables and their respective 95% confidence intervals (CIs) were determined using the binomial proportion. The area under the receiver operating characteristic curve (AUC) analysis was performed to evaluate the discriminatory ability of TCTA and CCTA for the diagnosis of significant coronary stenosis, as defined by CCA. Kappa (κ) statistical values were used to determine inter-observer agreement. A p value < 0.05 was considered to indicate significance for all analyses. All statistical analyses were performed using the SAS software, ver. 9.4 (SAS Institute Inc., Cary, NC, USA).
The study population consisted of 132 stroke patients and 164 non-stroke patients. The prevalence of atrial fibrillation was significantly higher in the TCTA group than in the CCTA group (10% vs. 2%, p = 0.003). Among 132 stroke patients, 25 carried a known CAD and 7 were diagnosed with peripheral artery occlusive disease. The other baseline clinical characteristics were not significantly different between the two groups (Table 1). Significant coronary stenoses on CCA and previous myocardial infarction (MI) on CT were found in 101 (76%) and 22 (17%) patients, respectively, in the TCTA group compared with 136 (83%) and 34 (21%) patients, respectively, in the CCTA group without significant differences between the two groups. There were also no significant differences in the proportion of single- and multi-vessel disease by CCA in both groups. The proportion of vessels with each lesion showed a similar distribution in both groups (Table 1). Among 28 (17%) non-stroke patients without significant coronary stenoses on CCA, 16 showed significant coronary stenoses on CCTA (false-positive results). However, 12 patients who showed insignificant coronary stenoses on CCTA underwent CCA because of patient complaints of typical angina and physicians were keen to avoid false-negative results. Among 31 (24%) stroke patients who showed no significant coronary stenoses on CCA, 17 manifested significant coronary stenoses on TCTA (false-positive results). However, 14 patients who showed insignificant coronary stenoses on TCTA underwent CCA because physicians were keen to avoid significant CAD (false-negative results).
Thoracic computed tomography angiography revealed the absence of intracardiac thrombus in all stroke patients, spontaneous echo contrast in LAA in 10 patients, akinetic or hypokinetic segmental myocardial wall in 24 patients (22 patients with old MI and 2 patients with dilated cardiomyopathy), interatrial septal aneurysm in 2 patients, patent foramen ovale in 1 patient, and atherosclerotic changes involving ascending thoracic aorta and aortic arch in 104 patients.
There were significant differences in average HR, image quality score of all coronary segments, image quality per coronary segment, mean radiation exposure to patients, and volume of administered contrast media between TCTA and CCTA (Table 2). We used the different acquisition protocol based on the patient's HR before CT scan. However, the patient's HR changed during CT scan. Therefore, the CT protocol based on HR was inappropriate in 31 (10%) patients (Table 3). ECG-based tube current modulation was used in 112 (85%) stroke patients and 159 (97%) non-stroke patients. Effective radiation dose between two groups varied significantly only at full-dose radiation for 65–75% of the R-R interval (TCTA group [5.6 ± 0.5 mSv] vs. CCTA group [4.8 ± 0.7 mSv], p < 0.0001).
Agatston coronary calcium score of the TCTA group was significantly lower than in the CCTA group (247 ± 247 vs. 387 ± 573, p = 0.01). One hundred fourteen (5.4%) coronary segments in stroke patients and 56 (2.1%) segments in non-stroke patients were excluded from the analysis. The reasons for exclusion were true absence of a vessel segment (n = 135) and small vessel size (n = 35). Of the remaining 1998 segments, 57 (2.9%) were considered non-evaluable on TCTA due to cardiac motion artifact (n = 27), severe calcification (n = 17), respiratory motion artifacts (n = 8), low vascular contrast (n = 4), and a stent (n = 1) (Fig. 2). There were 83 (3.2%) non-evaluable coronary segments on CCTA due to severe calcification (n = 64), cardiac motion artifacts (n = 12), respiratory motion artifacts (n = 5), and stents (n = 2). Non-evaluable coronary segments were not different between the two groups (p = 0.46). The κ values for inter-observer agreement of TCTA and CCTA for coronary segments were 0.82 (95% CI: 0.79–0.85) and 0.88 (95% CI: 0.86–0.91), respectively.
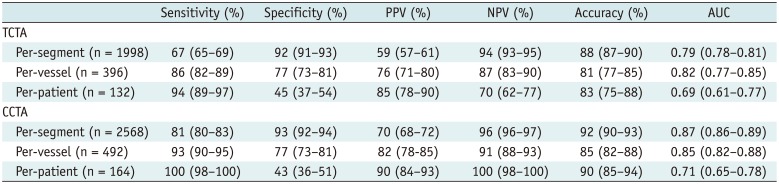
Significant stenosis was identified in 101 (76.5%) patients, 179 (45.2%) vessels, and 293 (14.7%) segments on CCA in the TCTA group. Significant stenosis was identified in 136 (82.9%) patients, 259 (52.6%) vessels, and 404 (15.7%) segments on CCA in the CCTA group. Sensitivity, specificity, PPV, NPV, and AUC for TCTA were 67%, 92%, 59%, 94%, and 0.79, respectively, on a per-segment basis; 86%, 77%, 76%, 87%, and 0.82, respectively, on a per-vessel basis; and 94%, 45%, 85%, 70%, and 0.69, respectively, on a per-patient basis (Table 4). Of the 57 segments not evaluated by TCTA, 10 segments with cardiac motion artifacts and 8 segments with severe calcification were significantly stenotic in CCA. Furthermore, 97 segments were identified as false-negative and associated with cardiac motion artifacts (n = 40), calcified plaques (n = 22), poor contrast enhancement (n = 17), measurement error (n = 11), and detection error (n = 7). On the other hand, 137 false-positive segments were identified, which were associated with cardiac motion artifacts (n = 65), calcified plaques (n = 28), poor contrast enhancement (n = 26), and measurement error (n = 18) (Fig. 2).
Sensitivity, specificity, PPV, NPV, and AUC for CCTA were 81%, 93%, 70%, 96%, and 0.87, respectively, on a per-segment basis; 93%, 77%, 82%, 91%, and 0.85, respectively, on a per-vessel basis; and 100%, 43%, 90%, 100%, and 0.71, respectively, on a per-patient basis (Table 4). Of the 83 segments non-evaluable by CCTA, 41 with severe calcification and 2 with cardiac motion artifacts were significantly stenotic in CCA. Furthermore, 75 segments were false-negative and associated with calcified plaques (n = 36), cardiac motion artifacts (n = 21), poor contrast enhancement (n = 9), measurement error (n = 6), and detection error (n = 3). On the other hand, 143 false-positive segments were associated with calcified plaques (n = 81), cardiac motion artifacts (n = 29), measurement error (n = 26), and poor contrast enhancement (n = 7). A significant difference existed in AUCs between TCTA and CCTA groups on per-segment basis (AUC of 0.79 vs. 0.87; p < 0.001).
There have been no studies to date assessing the diagnostic performance of ECG-gated TCTA to identify significant CAD in patients with ischemic stroke. In this study, ECG-gated TCTA without HR control facilitated identification of significant coronary stenosis in patients with acute ischemic stroke. TCTA was comparable to CCTA in the diagnosis of significant CAD even though inter-observer agreement and per-segment diagnostic performance of TCTA were lower than those of CCTA. Our experience indicates that ECG-gated TCTA evaluation can be performed with acceptable image quality at appropriate doses of radiation.
Coronary artery disease is considered a significant cause of morbidity and mortality in patients with ischemic stroke (23). The prevalence of asymptomatic but significant CAD is substantial in patients with ischemic stroke or transient ischemic attack (91011). Yoo et al. (11) showed that a substantial proportion (33.1%) of stroke patients who were not previously diagnosed with CAD had significant stenosis of at least one coronary artery on CCTA. Therefore, accurate noninvasive imaging can be used to detect high-risk cardiac sources of embolism and aortic atheroma. However, significant coronary stenoses are very important for prognostic evaluation and treatment planning in stroke patients. TEE is a semi-invasive imaging method for accurate and comprehensive evaluation of thrombi in the left atrium and its appendages as well as aortic atheroma (242526). Aortic atheroma evaluated with TEE has been correlated with a higher prevalence of CAD and the presence of significant angiographic coronary stenoses (27). For the last decade, a two-phase ECG-gated CCTA has been implicated as an accurate non-invasive imaging modality to discriminate thrombus from echo contrast within the LAA, in a manner that is comparable to TEE (2128). In this study, two-phase ECG-gated TCTA identified potential cardiac and aortogenic sources of emboli in patients with ischemic stroke. Accordingly, CCTA is a useful non-invasive imaging technique for the detection of cardiogenic emboli and significant CAD in stroke patients, particularly with the level of disability. However, CCTA only provides anatomic information of heart and proximal ascending thoracic aorta.
Triple rule-out CT is a useful imaging technique to identify life-threatening etiologies of chest pain, such as coronary stenoses, aortic dissection, and pulmonary embolism (2930). In groups matched for age, sex, body mass index, and HR, there were no significant differences between triple rule-out CT and dedicated CCTA using 64-slice multidetector CT in terms of overall image quality, presence of motion and streak artifacts, and contrast-to-noise ratio in the coronary lumen (31). Simultaneous evaluation of thoracic aorta and heart is also feasible with ECG-gated TCTA, such as triple rule-out CT with wide field of view from the aortic arch to the inferior border of the heart. ECG-gated TCTA increases the radiation exposure, and the amount and timing of contrast administration and image acquisition vary for CCTA. Evaluating patients with ischemic stroke in an acute setting is very challenging because they are likely to be old and exhibit fast or irregular HR and have trouble holding their breath during CT examination. In our hospital, beta-blockers are usually avoided in patients with acute ischemic stroke and first-generation 64-slice dual-source CT with contrast-enhancement, retrospectively. ECG-gated TCTA scan without HR control is performed to evaluate cardiac or aortic causes of stroke except in patients with poor breathing control. This approach may be not indicated for coronary evaluation in patients with ischemic stroke. However, with optimized cardiac phases, narrow field of view, and three-dimensional reconstruction of the heart from TCTA images, adequate images of the coronary arteries are obtained. In 100 patients not pretreated with beta-blockers, the first-generation dual-source CT demonstrated that 95% coronary vessels were evaluable in patients with a HR ≥ 65 bpm (mean 76 ± 9 bpm) but no significant decrease in diagnostic accuracy for detection of significant CAD between patients with low (< 65 bpm) and high HR (32). Therefore, we assumed that ECG-gated TCTA without HR control might be associated with satisfactory diagnostic performance in ischemic stroke patients similar to CCTA for detection of significant CAD in non-stroke patients with known or suspected CAD. Without use of beta-blockers for HR control, cardiac motion artifacts related to high HR or irregular cardiac rhythm deteriorate the image quality of a CT data set. Of the 25 non-evaluable segments with cardiac motion by TCTA, 10 (40%) were significantly stenotic at CCA. The ECG-gated TCTA protocol showed adequate image quality despite the lack of beta-blocker. Therefore, TCTA showed a similar diagnostic performance for detection of significant CAD at per-vessel and per-patient levels in ischemic stroke patients compared with CCTA in non-ischemic patients with known or suspected CAD. However, there was significant difference in AUCs between TCTA and CCTA groups on a per-segment basis. In addition, the inter-observer agreement on a per-segment basis for TCTA was lower than that of CCTA.
In this study, the tube voltage of 100 kV, a pitch automatically adapted to the HR and automatic tube current modulation, were used in all patients. Automatic ECG-pulsing with the Mindose protocol was used in patients with a mean HR < 90 bpm in regular heartbeat for radiation dose reduction. The radiation dose of TCTA protocol (6.8 mSv) was significantly higher than that of CCTA protocol (5.7 mSv). However, considering the wide scan range of combined arterial and delayed phases in the TCTA protocol compared with standard CCTA protocol, the radiation dose of TCTA protocol was not as high as expected. Radiation dose reduction in arterial phase of ECG-gated TCTA was related to higher HR (68 bpm) and lower tube current (90–120 mAs) compared with ECG-gated CCTA (61 bpm and 100–280 mAs, respectively). Among the three different CT protocols, the effective radiation dose between TCTA and CCTA groups was not significantly different at full-dose radiation for 20–70% of the R-R interval and no ECG-based tube current modulation was observed in patients with higher HR or irregular cardiac rhythm. Dual-source CT allows higher pitch values with increased HR, resulting in a decrease in radiation dose for the patient at increased pitch (33). In addition, radiation dose in our TCTA protocol was lower than in the previous study because of the use of ECG-based tube current modulation with the Mindose protocol, smaller scan range, and effective dose conversion coefficient for the chest (34).
There are no specific recommendations for coronary evaluation in ischemic stroke patients, with the exception of those individuals diagnosed with carotid artery disease or those at high risk based on Framingham risk score. These individuals warrant further CAD evaluation during their initial evaluation (23). Furthermore, few diagnostic imaging modalities can be effectively used for detection of CAD in stroke patients with physical disability. Accordingly, the ECG-gated TCTA protocol may be appropriate to detect CAD and high risk factors in cardiogenic embolism, and to evaluate the complex aortic plaques during hospitalization in ischemic stroke patients. A wide-detector CT or second- and third-generation dual-source CT offers greater potential to simultaneously assess the thoracic aorta and heart, as well as to optimize image quality compared with 64-slice CT or first-generation dual-source CT (35). These approaches may obviate the need for unnecessary further studies.
This study had several limitations. First, this is a single-institution retrospective study with a relatively small number of highly selected patients. Second, severe coronary calcification was the main cause of non-evaluable coronary segments and represented an important concern affecting the diagnostic performance of CCTA and TCTA for identification of significant CAD. Third, different study populations (stroke and non-stroke patients) were used to compare the two different CT protocols. The comparison group should be as similar to the study group as possible for comparative diagnostic accuracy between TCTA and CCTA for significant CAD. Fourth, the study results using a first-generation dual-source CT may not be applicable to other CT scanners with low temporal resolution. Fifth, the mean radiation exposure value for ECG-gated TCTA protocol was 6.8 ± 1.5 mSv. The radiation dose of ECG-gated TCTA may be decreased by reducing the tube current and tube voltage, and iterative reconstruction using cutting-edge CT scanners without compromising with the image quality. Finally, ECG-gated TCTA may have an inherent limitation for evaluation of coronary artery stenosis in patients with acute ischemic stroke because of differences in scanning protocol including contrast media injection, scan range, radiation dose, and HR control compared with ECG-gated CCTA.
In conclusion, ECG-gated TCTA reveals significant coronary stenosis in patients with acute ischemic stroke despite the absence of beta-blocker. Additional prospective studies with appropriate and larger patient cohorts are needed to assess the diagnostic accuracy of ECG-gated TCTA to identify significant CAD.
References
2. Ayala C, Greenlund KJ, Croft JB, Keenan NL, Donehoo RS, Giles WH, et al. Racial/ethnic disparities in mortality by stroke subtype in the United States, 1995-1998. Am J Epidemiol. 2001; 154:1057–1063. PMID: 11724723.

3. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993; 24:35–41. PMID: 7678184.

4. Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001; 32:2735–2740. PMID: 11739965.
5. Doufekias E, Segal AZ, Kizer JR. Cardiogenic and aortogenic brain embolism. J Am Coll Cardiol. 2008; 51:1049–1059. PMID: 18342221.

6. Hur J, Choi BW. Cardiac CT imaging for ischemic stroke: current and evolving clinical applications. Radiology. 2017; 283:14–28. PMID: 28318443.

7. Hur J, Kim YJ, Lee HJ, Ha JW, Heo JH, Choi EY, et al. Cardiac computed tomographic angiography for detection of cardiac sources of embolism in stroke patients. Stroke. 2009; 40:2073–2078. PMID: 19372451.

8. Hur J, Kim YJ, Lee HJ, Nam JE, Hong YJ, Kim HY, et al. Cardioembolic stroke: dual-energy cardiac CT for differentiation of left atrial appendage thrombus and circulatory stasis. Radiology. 2012; 263:688–695. PMID: 22495682.

9. Calvet D, Touzé E, Varenne O, Sablayrolles JL, Weber S, Mas JL. Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the PRECORIS study. Circulation. 2010; 121:1623–1629. PMID: 20351236.
10. Amarenco P, Lavallée PC, Labreuche J, Ducrocq G, Juliard JM, Feldman L, et al. Prevalence of coronary atherosclerosis in patients with cerebral infarction. Stroke. 2011; 42:22–29. PMID: 21088246.

11. Yoo J, Yang JH, Choi BW, Kim YD, Nam HS, Choi HY, et al. The frequency and risk of preclinical coronary artery disease detected using multichannel cardiac computed tomography in patients with ischemic stroke. Cerebrovasc Dis. 2012; 33:286–294. PMID: 22286013.

12. Brønnum-Hansen H, Davidsen M, Thorvaldsen P. Danish MONICA Study Group. Long-term survival and causes of death after stroke. Stroke. 2001; 32:2131–2136. PMID: 11546907.

13. Hartmann A, Rundek T, Mast H, Paik MC, Boden-Albala B, Mohr JP, et al. Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study. Neurology. 2001; 57:2000–2005. PMID: 11739816.

14. Hur J, Lee KH, Hong SR, Suh YJ, Hong YJ, Lee HJ, et al. Prognostic value of coronary computed tomography angiography in stroke patients. Atherosclerosis. 2015; 238:271–277. PMID: 25544177.

15. Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008; 52:2135–2144. PMID: 19095130.
16. Yoon YE, Lim TH. Current roles and future applications of cardiac CT: risk stratification of coronary artery disease. Korean J Radiol. 2014; 15:4–11. PMID: 24497786.

17. ASCI Practice Guideline Working Group. Beck KS, Kim JA, Choe YH, Hian SK, Hoe J, et al. 2017 multimodality appropriate use criteria for noninvasive cardiac imaging: expert consensus of the Asian society of cardiovascular imaging. Korean J Radiol. 2017; 18:871–880. PMID: 29089819.

18. Lee K, Hur J, Hong SR, Suh YJ, Im DJ, Kim YJ, et al. Predictors of recurrent stroke in patients with ischemic stroke: comparison study between transesophageal echocardiography and cardiac CT. Radiology. 2015; 276:381–389. PMID: 25692312.

19. Raff GL, Chinnaiyan KM, Share DA, Goraya TY, Kazerooni EA, Moscucci M, et al. Advanced Cardiovascular Imaging Consortium Co-Investigators. Radiation dose from cardiac computed tomography before and after implementation of radiation dose-reduction techniques. JAMA. 2009; 301:2340–2234. PMID: 19509381.

20. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for grading of coronary artery disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975; 51(4 Suppl):5–40. PMID: 1116248.

21. Choi BH, Ko SM, Hwang HK, Song MG, Shin JK, Kang WS, et al. Detection of left atrial thrombus in patients with mitral stenosis and atrial fibrillation: retrospective comparison of two-phase computed tomography, transoesophageal echocardiography and surgical findings. Eur Radiol. 2013; 23:2944–2953. PMID: 23821020.

22. Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009; 301:500–507. PMID: 19190314.

23. Adams RJ, Chimowitz MI, Alpert JS, Awad IA, Cerqueria MD, Fayad P, et al. Coronary risk evaluation in patients with transient ischemic attack and ischemic stroke: a scientific statement for healthcare professionals from the Stroke Council and the Council on Clinical Cardiology of the American Heart Association/American Stroke Association. Stroke. 2003; 34:2310–2322. PMID: 12958318.
24. Cujec B, Polasek P, Voll C, Shuaib A. Transesophageal echocardiography in the detection of potential cardiac source of embolism in stroke patients. Stroke. 1991; 22:727–733. PMID: 2057970.

25. Leung DY, Black IW, Cranney GB, Walsh WF, Grimm RA, Stewart WJ, et al. Selection of patients for transesophageal echocardiography after stroke and systemic embolic events. Role of transthoracic echocardiography. Stroke. 1995; 26:1820–1182. PMID: 7570732.
26. Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, Colonna P, et al. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr. 2010; 11:461–476. PMID: 20702884.

27. Fazio GP, Redberg RF, Winslow T, Schiller NB. Transesophageal echocardiographically detected atherosclerotic aortic plaque is a marker for coronary artery disease. J Am Coll Cardiol. 1993; 21:144–150. PMID: 8417055.

28. Hur J, Kim YJ, Lee HJ, Ha JW, Heo JH, Choi EY, et al. Left atrial appendage thrombi in stroke patients: detection with two-phase cardiac CT angiography versus transesophageal echocardiography. Radiology. 2009; 251:683–690. PMID: 19366905.

29. Halpern EJ. Triple-rule-out CT angiography for evaluation of acute chest pain and possible acute coronary syndrome. Radiology. 2009; 252:332–345. PMID: 19703877.

30. Burris AC 2nd, Boura JA, Raff GL, Chinnaiyan KM. Triple rule out versus coronary CT angiography in patients with acute chest pain: results from the ACIC consortium. JACC Cardiovasc Imaging. 2015; 8:817–825. PMID: 26093928.
31. Shapiro MD, Dodd JD, Kalva S, Wittram C, Hsu J, Nasir K, et al. A comprehensive electrocardiogram-gated 64-slice multidetector computed tomography imaging protocol to visualize the coronary arteries, thoracic aorta, and pulmonary vasculature in a single breath hold. J Comput Assist Tomogr. 2009; 33:225–232. PMID: 19346850.

32. Ropers U, Ropers D, Pflederer T, Anders K, Kuettner A, Stilianakis NI, et al. Influence of heart rate on the diagnostic accuracy of dual-source computed tomography coronary angiography. J Am Coll Cardiol. 2007; 50:2393–2398. PMID: 18154964.

33. Primak AN, McCollough CH, Bruesewitz MR, Zhang J, Fletcher JG. Relationship between noise, dose, and pitch in cardiac multi-detector row CT. Radiographics. 2006; 26:1785–1794. PMID: 17102050.

34. Feuchtner GM, Jodocy D, Klauser A, Haberfellner B, Aglan I, Spoeck A, et al. Radiation dose reduction by using 100-kV tube voltage in cardiac 64-slice computed tomography: a comparative study. Eur J Radiol. 2010; 75:e51–e56. PMID: 19671491.

35. Lell M, Hinkmann F, Anders K, Deak P, Kalender WA, Uder M, et al. High-pitch electrocardiogram-triggered computed tomography of the chest: initial results. Invest Radiol. 2009; 44:728–733. PMID: 19809339.
Fig. 1
Protocol for TCTA.
After coronary calcium scan, TCTA was acquired from aortic arch to inferior border of heart to detect significant coronary stenosis and high-risk sources of cardiogenic embolism, and to evaluate aortic plaques. Three minutes after TCTA, late-phase CT was acquired from left atrium to middle of left ventricle to distinguish between slow flow and thrombus in LAA without additional use of iodinated contrast medium. ECG = electrocardiogram, LAA = left atrial appendage, TCTA = thoracic computed tomography angiography
Fig. 2
Images in 70-year-old man with acute ischemic stroke.
Patient manifested hypertension, diabetes mellitus and atrial fibrillation. A. ECG obtained during TCTA examination showed atrial fibrillation and ventricular premature contraction. ECG-based tube current modulation was switched off in this patient. B. Curved multi-planar reformatted image shows approximately insignificant stenosis (arrowhead) via mixed calcified and non-calcified plaques and poor contrast enhancement (arrow) in PL. C. Curved multi-planar reformatted image shows poor contrast enhancement in middle segment of LAD (arrows), corresponding to non-evaluable segment. D. Curved multi-planar reformatted image shows poor contrast enhancement or diffuse obstruction in OM1 (arrows). Patient was diagnosed with 3-vessel disease by TCTA findings. E. Early-phase TCTA demonstrates triangular-shape filling defects within LAA (arrows). F. Delayed imaging reveals complete in-filling of appendage confirming that defect was secondary to circulatory stasis rather than thrombus. G. Oblique sagittal reconstruction image of aortic arch shows 3.5-mm-thick atheroma (arrow) of proximal aortic arch with hypoattenuating component. H–J. Conventional coronary angiography images showed no significant stenoses in early-branching PL (arrow, H) and middle LAD (arrow, I) whereas subtotal occlusion without collateral flow in OM1 (arrow, J). LAD = left anterior descending coronary artery, OM1 = first obtuse marginal artery, PL = posterolateral branch
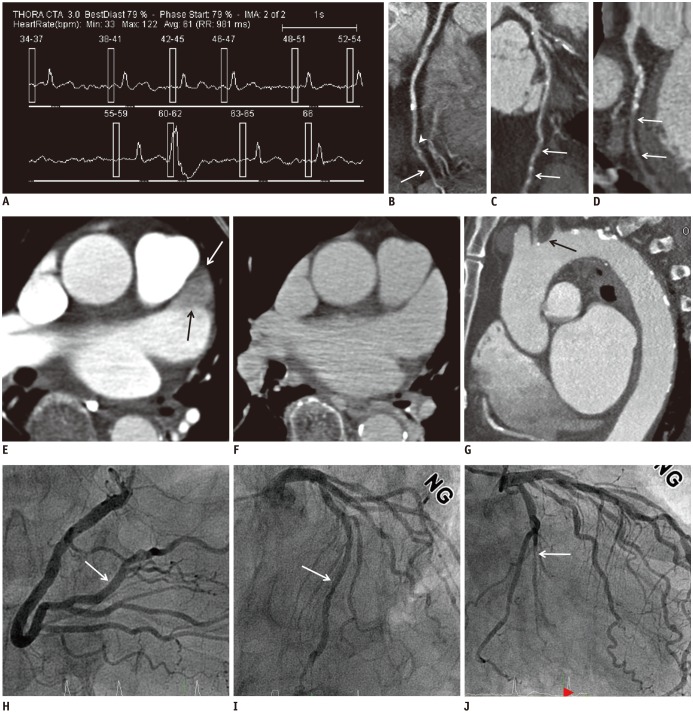
Table 1
Patient Characteristics
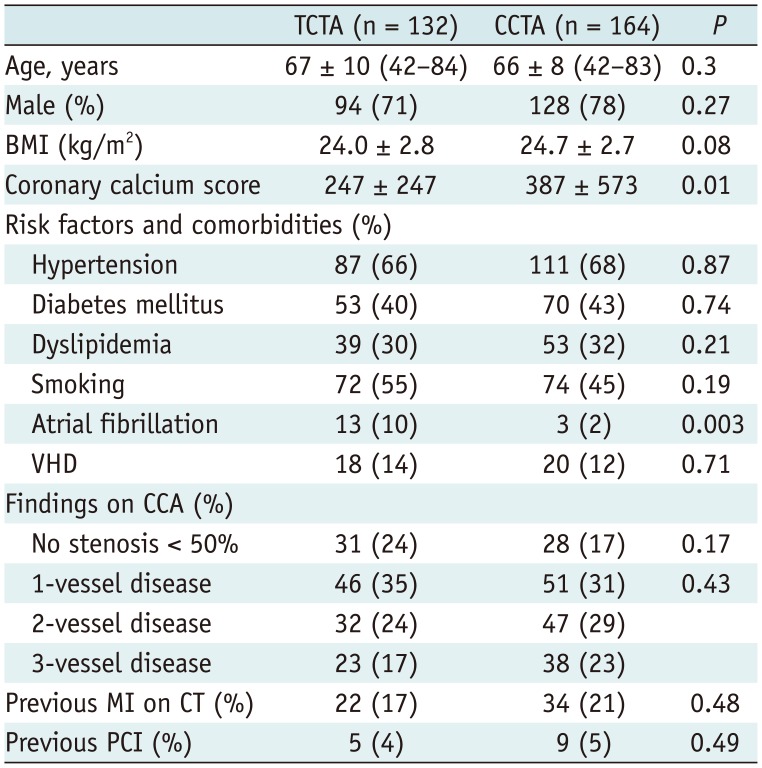
Table 2
Specifics of CT Examination Protocols
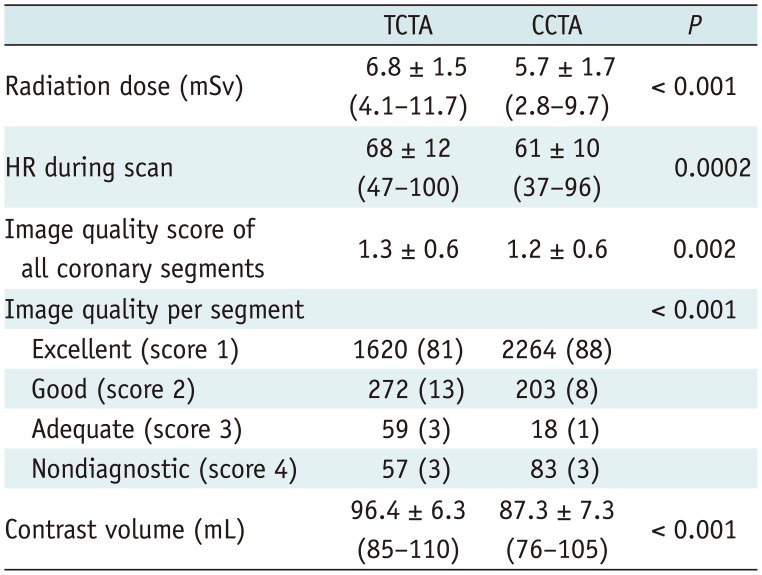
Table 3
Different CT Acquisition Protocol and Radiation Dose according to HR between ECG-Gated TCTA and CCTA Group
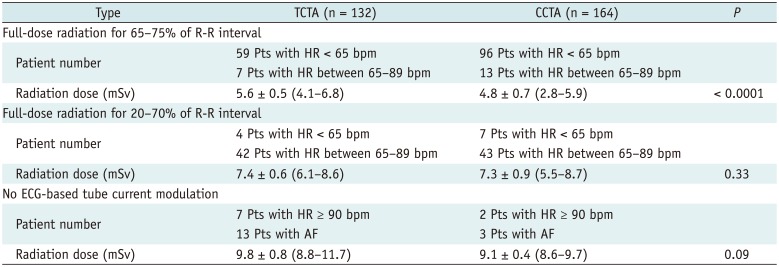
Table 4
Per-Segment, Per-Vessel Territory, and Per-Patient Diagnostic Accuracy of TCTA and CCTA Compared with CCA (Significant Stenosis ≥ 50%)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download