Abstract
Objective
We hypothesized that open bronchi within target pulmonary lesions are associated with percutaneous transthoracic needle biopsy (PTNB)-related hemoptysis. We sought to analyze and compare patient characteristics and target features as well as biopsy-related factors between patients with and without PTNB-related hemoptysis.
Materials and Methods
We retrospectively analyzed 1484 patients (870 males and 614 females; median age, 66 years) who had undergone 1569 cone-beam CT (CBCT)-guided PTNBs. Patient characteristics (sex, age, and pathologic diagnosis), nodule features (nodule type, size, location, and presence of an open bronchus in target nodules), and biopsy-related factors (biopsy needle size, pleura-to-target distance, blood test results, open bronchus unavoidability [OBU] index, etc.) were investigated. OBU index, which was assessed using the pre-procedural CBCT, was a subjective scoring system for the probability of needle penetration into the open bronchus. Univariate analysis and subsequent multivariate logistic regression analysis were conducted to reveal the independent risk factors for PTNB-related hemoptysis. For a subgroup of nodules with open bronchi, a trend analysis between the occurrence of hemoptysis and the OBU index was performed.
Results
The independent risk factors for hemoptysis were sex (female; odds ratio [OR], 1.918; p < 0.001), nodule size (OR, 0.837; p < 0.001), open bronchus (OR, 2.101; p < 0.001), and pleura-to-target distance (OR, 1.135; p = 0.003). For the target nodules with open bronchi, a significant trend between hemoptysis and OBU index (p < 0.001) was observed.
Lung cancer is the leading cause of cancer death in both males and females, accounting for more than 25% of estimated cancer deaths (1). It usually manifests as a pulmonary nodule on chest radiographs or computed-tomography (CT) examinations. The initial diagnostic approach is based on the pretest probability of cancer according to the patient's clinical risk factors and CT features (23). Invasive diagnostic procedures such as percutaneous transthoracic needle biopsy (PTNB) are often inevitable for a confirmative diagnosis in patients with suspicious malignant lung nodules, particularly those with an intermediate risk of malignancy (23).
Imaging-guided PTNB is one of the most widely utilized diagnostic methods with an accuracy of greater than 90% for the histopathologic diagnosis of a lung nodule (4). This invasive procedure is necessarily accompanied by a number of complications, such as pneumothorax and hemoptysis (5). PTNB-related hemoptysis is usually self-limiting, and amenable to conservative management (5). However, it may be fatal for elderly patients with certain comorbid conditions, such as congestive heart failure or pulmonary fibrosis, which result in hypoxemia. Therefore, prediction of hemoptysis before PTNBs is important for interventional radiologists as well as for clinicians and facilitates determination of the inclusion or exclusion of patients at risk. For patients with substantial risk, alternatives such as endobronchial ultrasound-guided biopsy or surgical resection may be recommended (6). Several risk factors for hemoptysis have been reported in the literature, including the target size, density, and depth of target lesions (578).
The presence of open-bronchus sign within target pulmonary lesions is a potential risk factor in PTNB-related hemoptysis in that internal open bronchi are directly connected to the central airways, and the hollow cylindrical structure of an open bronchus may not act as a tamponade against PTNB-related bleeding. However, little is known regarding the role of open or patent bronchus within target pulmonary lesions in PTNB-related hemoptysis. In this study, we hypothesized that open bronchi within target pulmonary lesions are associated with PTNB-related hemoptysis. The purpose of this study was to analyze and compare patient characteristics and target features as well as biopsy-related factors between patients with and without PTNB-related hemoptysis.
This retrospective analysis was approved by the Institutional Review Board of Seoul National University Hospital, and the requirement for written informed consent was waived.
Between August 2015 and May 2017, 1743 consecutive patients underwent PTNBs under the guidance of cone-beam CT (CBCT) at a single tertiary medical center. PTNBs were performed based on the clinical indications, technical feasibility, and consideration of benefits and harms from the procedures. Among the 1743 patients, 259 patients were excluded, since their target lesions were not located in the lung (n = 66; 41 in the mediastinum, 21 in the pleura, and 4 in the chest wall), or their clinical characteristics or biopsy-related factors were not derived from the electronic medical records (EMRs) or picture archiving and communication system (n = 193). Finally, 1484 patients (median age, 66 years; interquartile range [IQR], 57–74 years) were included in this study. Among these patients, 85 underwent repeat biopsies, and thus, a total of 1569 PTNBs were analyzed in the present study. There were 870 males (median age, 68 years; IQR, 59–75 years) and 614 females (median age, 62 years; IQR, 54–70 years). Target pulmonary lesions had a median diameter of 2.7 cm (IQR, 1.8–4.2 cm). A few of the study subjects (199/1484) were previously reported (9).
All biopsy procedures were performed by or under the supervision of one of the two experienced chest radiologists (with 11 years and 4 years of experience in image-guided thoracic interventions, respectively) using two different CBCT systems (Artis Zee, Siemens Healthineers, Forchheim, Germany; Allura Xper FD20, Philips Healthcare, Best, the Netherlands).
Pre-procedural CBCT was used to define the needle path using virtual navigation software programs (Syngo i-Guide, Siemens Healthineers; XperGuide, Philips Healthcare), and intraprocedural CBCT was used to confirm the needle tip location in the targets. Post-procedural CBCT was also used to evaluate any immediate biopsy-related complications. Coaxial core biopsies were performed with 17-gauge introducers and 18-gauge cutting biopsy needles, or with 18-gauge introducers and 20-gauge cutting needles (Stericut, TSK Laboratory, Tochigi-ken, Japan). Aspiration biopsy was performed with 22-gauge needles (Westcott, Angiotech, FL, USA). Our routine biopsy protocol entailed a core biopsy for the acquisition of two or three specimens. Aspiration was an option in case of extensive necrotic mass or a risky lesion for core biopsy. Details of our CBCT-guided PTNB procedures have been described in previous publications (71011).
A complete blood count and coagulation profile were obtained prior to the procedure in all cases. A biopsy was deferred for patients with a platelet count of ≤ 70000/mm3, a prothrombin time-to-international normalized ratio (PT INR) ≥ 1.5, or an activated partial thromboplastin time (aPTT) ≥ 50 seconds. In our institution, clopidogrel was held for at least 7 days prior to PTNBs, and acetylsalicylic acid (ASA) was discontinued for at least 3 days prior. However, continued ASA usage was not a contraindication.
Patient characteristics such as sex, age, and a pathologic diagnosis of the biopsy specimen were collected from the EMRs. All nodule features and biopsy-related factors were acquired from the operators' reports, a structured reporting system for PTNB procedures (71011). The following nodule features and biopsy-related factors were obtained: nodule type (solid or subsolid), nodule size (longest diameter), nodule location (lower lobes or others), number of intraprocedural CBCT scans, presence of emphysema along the biopsy needle track (yes or no), presence of an open bronchus within a target nodule (yes or no), patient position (prone or supine), biopsy needle size (18-G or smaller gauge [20 and 22]), number of pleural passages, number of core biopsies, confirmation of needle tip-in-target at procedural CBCT (yes or no), initial or repeat biopsy, pleura-to-target distance, needle indwelling time, total procedural time, blood test results (platelet count, PT INR, and aPTT), and occurrence of hemoptysis (yes or no). Hemoptysis encompassed a broad spectrum of conditions from blood mixed in phlegm to active coughing up of blood (8). Biopsy method (aspiration biopsy or core biopsy) was not included as only a limited number of aspiration biopsies (n = 6) were performed in this study. Instead, aspiration biopsy was counted in the number of core biopsies as ‘0.’
In the subgroup analysis, an open bronchus unavoidability index (OBU index), which described the operators' subjective scoring from 1 to 5 with respect to the probability of needle advancement or penetration into the open bronchus, was developed and recorded (1, none; 2, low; 3, possible; 4, probable; 5, high) (Fig. 1). OBU index was analyzed using the pre-procedural CBCT scans.
To reveal the risk factors for hemoptysis in CBCT-guided PTNBs, univariate analysis was initially performed. Patient characteristics, nodule features, and biopsy-related factors were compared between patients with and without hemoptysis after the PTNBs. A Pearson chi-squared or Fisher's exact test was used for categorical variables, and a Mann-Whitney U test was used for continuous variables as appropriate.
Subsequently, a multivariate logistic regression analysis was conducted to identify independent risk factors for hemoptysis with input variables, which showed p values < 0.05 at univariate analysis. Occurrence of hemoptysis was the dependent variable. Multiple logistic regression analysis was conducted using a backward elimination mode, with iterative entry of variables based on test results (p < 0.05). The elimination of variables was based on likelihood ratio statistics with a probability of 0.10.
In a subgroup of patients whose target nodules showed an open bronchus, we performed a trend analysis of the occurrence of hemoptysis based on the OBU index by using a Mantel-Haenszel linear-by-linear association test (12). We sought to determine the utility of subjective imaging assessments of radiologists before procedures in patients at risk of hemoptysis.
Statistical analyses were performed on a per-PTNB case basis and at a significance level of 0.05, using SPSS 19.0 (IBM Corp., Armonk, NY, USA).
Hemoptysis occurred in 174 out of 1569 PTNBs (11.1%). In these cases, the patients were immediately placed in the decubitus position with the biopsy side down, and oxygen was supplied via a nasal prong or a facial mask. Patients were managed conservatively with active expectoration of blood. Surgical intervention or endovascular embolization was not performed. There was no mortality associated with hemoptysis in this study population.
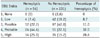
Univariate analysis (Tables 1, 2) revealed significant differences in sex (p < 0.001), target nodule type (p = 0.002), nodule size (p < 0.001), the presence of an open bronchus within the target nodules (p < 0.001), and pleura-to-target distance (p < 0.001) between patients with and without hemoptysis. Other variables such as number of core biopsies (p = 0.036), needle indwelling time (p = 0.010), and total procedural time (p < 0.001) also were significantly different between the two groups. In contrast, patient age, nodule location in lower lobes, pathological diagnosis, number of intraprocedural CBCT scans, emphysema along the needle track, patient position, needle size, number of pleural passages, biopsy needle tip-in-target, repeat biopsy, and blood test results (platelet count and coagulation profile) were not significantly associated with any group of patients (p > 0.05).
Multivariate logistic regression analysis of input variables such as sex, nodule type, nodule size, presence of an open bronchus, and pleura-to-target distance was performed. The number of core biopsies was not included, as the small number of biopsies associated with hemoptysis was caused by the cessation of the biopsy due to intra-procedural hemoptysis. The needle indwelling time or total procedural time was also not included. Needle indwelling time could not be predicted as a risk factor before the biopsy and was caused by other confounders (nodule size or depth). Increased total procedural time was considered a consequence of hemoptysis during biopsy.
Upon multivariate analysis (Table 3), we found that female sex (odds ratio [OR], 1.918; p < 0.001), smaller nodule size (OR, 0.837; p = 0.001), an open bronchus within a target nodule (OR, 2.101; p < 0.001), and longer pleura-to-target distance (OR, 1.135; p = 0.003) were independent risk factors for PTNB-related hemoptysis. A Hosmer-Lemeshow goodness-of-fit test showed that the model fit was appropriate (p = 0.149).
Among 285 PTNB cases in which the target nodules had open bronchi, 18.9% (54/285) manifested hemoptysis. There was a significant trend between hemoptysis and the OBU index (p < 0.001) (Table 4, Figs. 2, 3). The percentage of hemoptysis was highest in patients with an OBU index 4 (32.0%), followed by OBU indices 5 (28.6%), 3 (11.0%), 2 (8.7%), and 1 (0%).
In this study, we found that the open bronchus in a target nodule was an independent risk factor for PTNB-related hemoptysis. Subjective imaging assessment of OBU index showed a significant trend involving PTNB-related hemoptysis. Therefore, the OBU index was useful in the prediction of hemoptysis among patients with open bronchi. Several other independent risk factors for hemoptysis were female sex, smaller nodule size, and longer pleura-to-target distance.
An open or patent bronchus in a target nodule is frequently observed, and 18.2% (285/1569) of the target lesions in our study showed this radiologic feature. Intratumoral tissue or vascular injury leading to intratumoral hemorrhage occurs during tissue sampling process, particularly with core biopsies. In such a situation, the direct connection between an intratumoral open bronchus and the central airways induces hemoptysis. Since this open bronchus is hollow inside, it is also hard to expect a tamponade effect via the surrounding dense tumor tissue during bleeding following penetration or puncture of this intratumoral open bronchus by a biopsy needle. Therefore, whenever possible, the intratumoral area with a patent bronchus should be avoided as a tissue sampling site. To our knowledge, this is the first study to report the significance of an open bronchus within a target lesion with respect to PTNB-related hemoptysis.
Interestingly, a meticulous imaging evaluation of a target lesion prior to biopsy enabled the operators to score the potential risk of an open bronchus for hemoptysis. We assume that our findings can assist radiologists in performing biopsies in patients at risk. Patients with an impaired coagulation status or patients suffering from hypoxemia caused by single-lung ventilation or chronic obstructive pulmonary disease may not be able to tolerate hemoptysis and the accompanying respiratory distress. In these patients, a careful risk-benefit analysis of PTNB is mandatory. Diagnostic strategy should be tailored individually based on the potential risks and benefits. If needle penetration into the open bronchus is inevitable and consequent hemoptysis is expected, other options such as close surveillance or direct surgical resection should be considered.
Multiple other independent risk factors for hemoptysis include: female sex, smaller target lesion size, and longer pleura-to-target distance. These features have been reported repeatedly in the literature as risk factors for pulmonary hemorrhage or hemoptysis after PTNBs (578131415). In a recent study by Wang et al. (8), small lesion size (2–3 cm; OR, 3.22), longer pleura-to-target distance (intrapulmonary length of needle path > 2 cm; OR, 8.85), and proximity of the needle path to the pulmonary artery (OR, 10.33) were independent risk factors for hemoptysis after CT-guided PTNBs. In CBCT-guided PTNBs, Lee et al. (7) reported that nodule type (subsolid nodule; OR, 4.460) and pleura-to-target distance (OR, 1.297) were the risk factors for hemoptysis. In addition, Song et al. (5) demonstrated that dual-antiplatelet therapy (OR, 10.09), female sex (OR, 1.88), small lesion size (OR, 0.88), longer pleura-to-target distance, and the use of a cutting biopsy needle (OR, 3.22) were significant predictors. Interestingly, female sex as a potential risk factor for hemoptysis or pulmonary hemorrhage was reported by at least two studies (1416). We suppose that there may be a potentially confounding variable such as histopathologic diagnosis of the lesion in females as a risk factor. Nevertheless, it is currently difficult to explain the link between female sex and PTNB-related hemoptysis. As for the size, the small lesion may require more frequent needle re-direction during lesion targeting. Thus, the chance of bronchovascular injury is higher for small lesions. In addition, longer distance from pleura to the target as a risk factor is understandable in a similar context. Longer needle passage through pulmonary parenchyma necessarily induces bronchovascular bundle injury more frequently.
The frequency of hemoptysis in our study population was 11.1%. The rate of biopsy-related hemoptysis in PTNB ranged from 1.8% to 20.6% in previous studies (578111417181920212223). This wide range of hemoptysis may be attributed to the variety of biopsy methods available, operator experience, or the definition of hemoptysis among the studies (578111417181920212223). When the studies were limited to CBCT-guided PTNB, the frequency of hemoptysis was 2.0–14.5% (7111819202123). The rate of hemoptysis in our study was relatively close to the upper border of the reported range, and the possible reasons included the usage of cutting needle biopsy as a procedural standard, the acquisition of multiple core biopsy specimens (median, 3), and the small lesion size (median, 2.7 cm) in our patients. In addition, in our study, hemoptysis was defined by a broad range of manifestations from blood-tinged sputum to blood expectoration.
There were several limitations to this study. First, we did not evaluate the detailed medication history (dosage and duration of usage) including treatment with anticoagulants and antiplatelet agents prior to the PTNBs. However, in our institution, patients with coagulation problems were routinely monitored carefully prior to PTNBs. Any coagulation problems were corrected. Otherwise, patients with uncorrected coagulation problems were excluded from the PTNBs. Second, we did not perform subgroup analysis according to the amount of hemoptysis, as this was not recorded in our hospital's database. Massive hemoptysis is a life-threatening condition with disastrous outcomes. Therefore, the prediction of massive hemoptysis with clinical and radiologic features before biopsy is of substantial clinical value. The causal relationship between open bronchus and massive hemoptysis should be explored in a larger study population in the future. Third, delayed hemorrhage or hemoptysis was not investigated in the present study. Lastly, a caveat should be borne in mind that the open bronchus sign was an independent risk factor for, but not a sole determinant of hemoptysis. A majority of patients (69%) who manifested hemoptysis did not show open bronchus sign implying a multifactorial etiology of biopsy-related hemoptysis. In conclusion, an open bronchus in a biopsy target as well as female sex, smaller nodule size, and longer pleura-to-target distance are independent risk factors, and careful imaging review prior to biopsy has the potential to reduce the risk of PTNB-related hemoptysis.
Figures and Tables
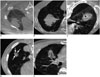 | Fig. 1OBU index.
A. 1 (none): single open bronchus (arrow) in tumor periphery. B. 2 (low): multiple open bronchi in tumor periphery with adequate room to advance cutting needle into tumor. C. 3 (possible): centrally (arrow) and peripherally located open bronchi in tumor with space to advance cutting needle into tumor while evading open bronchi. D. 4 (probable): it is possible that open bronchi (arrows) in tumor are injured or penetrated by cutting needle. E. 5 (high): it is highly likely that open bronchi in tumor are penetrated by cutting needle. OBU = open bronchus unavoidability
|
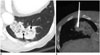 | Fig. 277-year-old male with invasive mucinous adenocarcinoma demonstrating extensive open bronchi.
A. Extensive open bronchi (arrows) were observed on axial CT scan, and OBU index was 5 (high). B. Biopsy needle penetrated open bronchi, and patient had immediate hemoptysis following core biopsy.
|
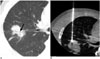 | Fig. 363-year-old male with invasive adenocarcinoma showing open bronchus.
A. Open bronchus (arrow; OBU index, 3) was noted within tumor, connected to central airway. B. Therefore, inferior portion of tumor, which was solid and without patent bronchus, was targeted. Pre-procedural imaging evaluation and needle path planning facilitated prevention of hemoptysis.
|
Table 1
Comparison of Clinical Characteristics between Patients with and without Hemoptysis after CBCT-Guided PTNB
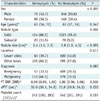
Unless otherwise specified, data are numbers of patients (with percentages in parentheses). *Data are median (with IQR in parentheses). aPTT = activated partial thromboplastin time, CBCT = cone-beam computed tomography, IQR = interquartile range, PT INR = prothrombin time-to-international normalized ratio, PTNB = percutaneous transthoracic needle biopsy
Table 2
Comparison of Biopsy-Related Characteristics between Patients with and without Hemoptysis after CBCT-Guided PTNB
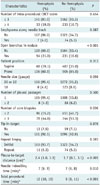
Table 3
Multivariate Logistic Regression Analysis for Prediction of PTNB-Related Hemoptysis

References
2. Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015; 70:Suppl 2. ii1–ii54.
3. Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med. 2012; 185:363–372.

4. Rotolo N, Floridi C, Imperatori A, Fontana F, Ierardi AM, Mangini M, et al. Comparison of cone-beam CT-guided and CT fluoroscopy-guided transthoracic needle biopsy of lung nodules. Eur Radiol. 2016; 26:381–389.

5. Song YS, Park CM, Park KW, Kim KG, Lee HJ, Shim MS, et al. Does antiplatelet therapy increase the risk of hemoptysis during percutaneous transthoracic needle biopsy of a pulmonary lesion? AJR Am J Roentgenol. 2013; 200:1014–1019.

6. Lee BE, Kletsman E, Rutledge JR, Korst RJ. Utility of endobronchial ultrasound-guided mediastinal lymph node biopsy in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. 2012; 143:585–590.

7. Lee SM, Park CM, Lee KH, Bahn YE, Kim JI, Goo JM. C-arm cone-beam CT-guided percutaneous transthoracic needle biopsy of lung nodules: clinical experience in 1108 patients. Radiology. 2014; 271:291–300.

8. Wang Y, Jiang F, Tan X, Tian P. CT-guided percutaneous transthoracic needle biopsy for paramediastinal and nonparamediastinal lung lesions: diagnostic yield and complications in 1484 patients. Medicine (Baltimore). 2016; 95:e4460.
9. Lim WH, Park CM, Yoon SH, Lim HJ, Hwang EJ, Lee JH, et al. Time-dependent analysis of incidence, risk factors and clinical significance of pneumothorax after percutaneous lung biopsy. Eur Radiol. 2018; 28:1328–1337.

10. Choo JY, Park CM, Lee NK, Lee SM, Lee HJ, Goo JM. Percutaneous transthoracic needle biopsy of small (≤ 1 cm) lung nodules under C-arm cone-beam CT virtual navigation guidance. Eur Radiol. 2013; 23:712–719.
11. Jin KN, Park CM, Goo JM, Lee HJ, Lee Y, Kim JI, et al. Initial experience of percutaneous transthoracic needle biopsy of lung nodules using C-arm cone-beam CT systems. Eur Radiol. 2010; 20:2108–2115.

12. Saba L, Montisci R, Raz E, Sanfilippo R, Suri JS, Piga M. Association between carotid artery plaque type and cerebral microbleeds. AJNR Am J Neuroradiol. 2012; 33:2144–2150.

13. Khan MF, Straub R, Moghaddam SR, Maataoui A, Gurung J, Wagner TO, et al. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol. 2008; 18:1356–1363.

14. Tai R, Dunne RM, Trotman-Dickenson B, Jacobson FL, Madan R, Kumamaru KK, et al. Frequency and severity of pulmonary hemorrhage in patients undergoing percutaneous CT-guided transthoracic lung biopsy: single-institution experience of 1175 cases. Radiology. 2016; 279:287–296.

15. Yeow KM, See LC, Lui KW, Lin MC, Tsao TC, Ng KF, et al. Risk factors for pneumothorax and bleeding after CT-guided percutaneous coaxial cutting needle biopsy of lung lesions. J Vasc Interv Radiol. 2001; 12:1305–1312.

16. Yildirim E, Kirbas I, Harman A, Ozyer U, Tore HG, Aytekin C, et al. CT-guided cutting needle lung biopsy using modified coaxial technique: factors effecting risk of complications. Eur J Radiol. 2009; 70:57–60.

17. Anzidei M, Sacconi B, Fraioli F, Saba L, Lucatelli P, Napoli A, et al. Development of a prediction model and risk score for procedure-related complications in patients undergoing percutaneous computed tomography-guided lung biopsy. Eur J Cardiothorac Surg. 2015; 48:e1–e6.

18. Cheng YC, Tsai SH, Cheng Y, Chen JH, Chai JW, Chen CC. Percutaneous transthoracic lung biopsy: comparison between C-arm cone-beam CT and conventional CT guidance. Transl Oncol. 2015; 8:258–264.

19. Choi MJ, Kim Y, Hong YS, Shim SS, Lim SM, Lee JK. Transthoracic needle biopsy using a C-arm cone-beam CT system: diagnostic accuracy and safety. Br J Radiol. 2012; 85:e182–e187.

20. Jaconi M, Pagni F, Vacirca F, Leni D, Corso R, Cortinovis D, et al. C-arm cone-beam CT-guided transthoracic lung core needle biopsy as a standard diagnostic tool: an observational study. Medicine (Baltimore). 2015; 94:e698.
21. Jiao de C, Li TF, Han XW, Wu G, Ma J, Fu MT, et al. Clinical applications of the C-arm cone-beam CT-based 3D needle guidance system in performing percutaneous transthoracic needle biopsy of pulmonary lesions. Diagn Interv Radiol. 2014; 20:470–474.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download