Abstract
Organoid is an in vitro multicellular form mimicking in vivo organ. Its similarity to human organ including cellular organization, molecular expression patterns, as well as genetic signatures enables to study the characteristics of infectious agents and host-pathogen interaction. For the features of organoid, this system also can be potentially used to cultivate currently uncultivable viruses of vaccine candidates. This paper will briefly describe problems in the current culture system for virus production and the possibility of organoid as culture system for viral vaccine and their current limitations that should be solved to meet the goal.
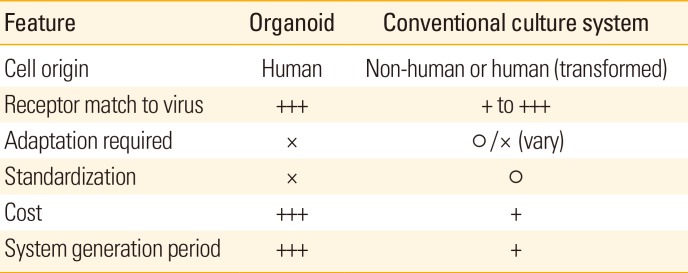
Inactivated vaccines have been widely used as viral vaccines because the vaccine formulation contains whole antigenic components and original shape of the pathogens. Despite of these merits, there are limitations for production of many viral vaccine strains and considering points in current virus producing system (Table 1): (1) insufficient production efficiency of vaccine strain, (2) genetic mutations during adaptation process, and (3) potential risk of non-human protein derived from virus replicating host cells. Rotavirus, norovirus, and hepatitis C virus (HCV) are representatives of uncultivable viruses and therefore having problem for vaccine production. These viruses do not have infectivity for animal cells used in virus production usually, transformed human cells, and even small-animal or grow very slowly even after adaptation process in the cell partly due to receptor mismatch and other intrinsic factor for specifically influencing replication in human cells [12345]. To generate, to test, and to use as a vaccine for these kinds of viruses, novel system for virus culture is mandatory. In case of low replicative viruses in the current in vitro culture system, adaptation process is widely used to increase the rate of virus entry and replication. During adaptation process, viral genomes are usually mutated. For example, influenza A virus PR8 have changed in 19 nucleotides, resulted in 13 amino acid changes after 20 passage of adaptation in Vero cell [6]. Dengue virus type 4 also has eight missense mutations and eight amino acid changes after adaptation in Vero cells [7]. These kinds of genetic mutation and resulting changes in amino acid sequences may potentially influence its immunogenicity as well as specificity and neutralizing activity of the induced virus-specific antibodies. Many vaccine strains of viruses are produced in non-human cell system and the representative is influenza virus which is grown in an allantoic fluid in embryonated chicken eggs [8]. Japanese encephalitis virus also produced in non-human cell culture system; mouse brain for Nakayama-NIH or Beijing-1 (P-1) strains, primary hamster kidney cell for Beijing-3 (P-3) strain, and Vero cell for P-1, P-3 or SA14-14-2 strains [9]. In these cases, there is potential risk to induce adverse effect by the host cell-derived proteins in the vaccine. Thus, human-cell based and highly efficient virus production system is urgently required to bring more effective viral vaccines that are currently unavailable.
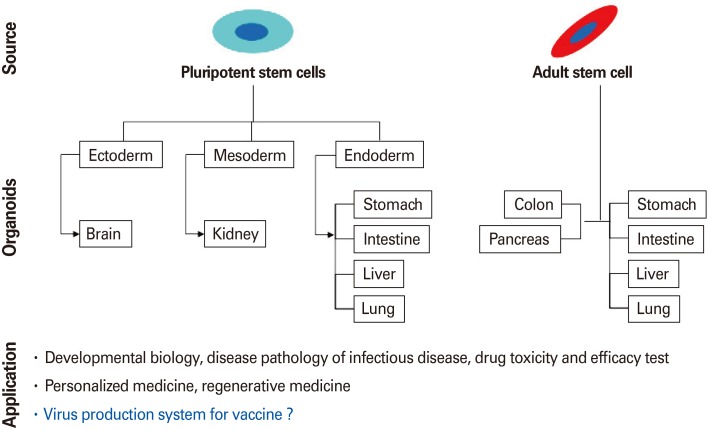
Organoid is stem cell derived multicellular self-organizing organ (Fig. 1) [10]. The major characteristic of organoid is mimicking the organization, functionality, and genetic signature of the specific tissue or organ in vivo. These features provide organoids with a potential to be used as a model to study infectious pathogens in terms of characteristics of the pathogens as well as host-pathogen interaction. Up to date, there are several organoids have been established such as intestine, stomach, esophagus, liver, kidneys, lungs, brain, prostate, pancreas, retina, and ovary [11]. Intestinal organoid is most well-known organoid type which is established with the successful culture of murine epithelial organoids from Lgr5+ intestinal stem cells in 2009, followed by the development of organoids from human pluripotent stem cells (PSCs) and biopsy samples [1213]. There are two-types of intestinal organoid: human intestinal organoid (HIO) and human intestinal enteroid (HIE). HIO is derived from PSCs that are epithelial cultures associated with mesenchyme. HIE is human tissue-derived organoids that are epithelial only cultures derived stem cells isolated from biopsies or surgical tissues. Using HIO model, rotavirus tropism, replication in enterocytes as well as mesenchymal cells, was identified [14]. Under HIE model, critical component for norovirus replication, bile 9a, was found [15]. Other organoids are also intensely used as infection models to investigate virus studies (cerebral organoid for Zika virus and lung organoid for respiratory syncytial virus) [161718].
Based on the in vivo mimicking features of organoids, there are some trials to use organoid as virus production system. Finkbeiner et al. [14] generated HIO system using embryonic stem cell line (WA09) and tested whether this system is suitable to cultivate human rotavirus. The generated HIO system successfully produced viral RNA and viroplasms after infection with clinically isolate samples collected from stool. Importantly, rotavirus replicated approximately 10 times higher in HIO system compared to conventional culture method using monkey epithelial cell line. Ettayebi et al. [15] tested HIE system as a cultivation system for human norovirus. In HIE system, human norovirus replicated time-dependent manner between 1- and 24-hour post infection (hpi). Genomic equivalent of viral progeny increased up to 1.5 to 2.5 log10 at 96 hpi compared to 1 hpi, suggesting that stem-cell derived intestinal organoid can be applicable to cultivate previously non-cultivatable human norovirus. This HIE system also suitable to assess virus neutralization. Although not actual organoid, three-dimensional (3-D) culture of Huh-7 showed the potential to cultivate HCV [19]. 3-D rotating wall vessel-culture method made Huh-7 cells form complex, multilayered, 3-D aggregates which is organoid-like phenotype. The cultured 3-D Huh-7 cells expressed high levels of metabolic-associate molecules (cytochrome P450s and UDP-glucuronosyltransferase), hepatocyte specific transcription factor (hepatocyte transcription factor), markers for re-localization of tight junction (Claudin-1, ZO-1, and Occludin-1), cell adhesion (E-cadherin and β-catenin), and polarity (CD26), and HCV receptor (CD81 and Scavenger receptor class B type 1). 3-D Huh-7 aggregates also showed localization of tight junction markers to apicalateral and/or basolateral planes which are consistent with localization pattern in primary hepatocytes and not observed in 2-D Huh-7. Most important, 3-D Huh-7 system was suitable for HCV infection and the production of HCV was exponentially increased, suggesting the potential of this new culture system for efficient HCV production. As described above, initial studies using organoids have been focused on the improving cultivation efficiency of currently uncultivable viruses. Given that the organoids are derived from human cells, this viral cultivation system does not need an adaptation process of viruses, indicating that there is no or relatively low potential to induce genetic mutation in the viral genome. In addition, there is no potential risk of adverse effect by host cell-derived non-human protein, which can be another merit of organoid system as a cultivation system of vaccine strains.
Although the potential of organoid in terms of virus producing efficiency was not verified in other various kind of virus, the initial trials suggested the possibility that organoid-based viral vaccine production can be more widely used in more broad ranges of viruses. To use organoid as a productive system for viral vaccine strain, there are some hurdles that remain to be solved (Table 1). The source of organoid and the generated organoid should be standardized to secure the quality of the system. In addition, the virus producing scheme under organoid system should be optimized to produce virus sufficiently without induction of genetic mutations in virus. And the cultivation and maintaining cost for organoid system also should be reduced to the degree comparable to that of conventional virus culture method. Technical advances to solve these issues will bring us more various and effective vaccines for viral infection.
References
1. Ciarlet M, Estes MK, Barone C, Ramig RF, Conner ME. Analysis of host range restriction determinants in the rabbit model: comparison of homologous and heterologous rotavirus infections. J Virol. 1998; 72:2341–2351. PMID: 9499095.

2. Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996; 77(Pt 7):1431–1441. PMID: 8757984.

3. Hoshino Y, Saif LJ, Kang SY, Sereno MM, Chen WK, Kapikian AZ. Identification of group A rotavirus genes associated with virulence of a porcine rotavirus and host range restriction of a human rotavirus in the gnotobiotic piglet model. Virology. 1995; 209:274–280. PMID: 7747480.

4. Kitamoto N, Ramig RF, Matson DO, Estes MK. Comparative growth of different rotavirus strains in differentiated cells (MA104, HepG2, and CaCo-2). Virology. 1991; 184:729–737. PMID: 1653495.

5. Superti F, Tinari A, Baldassarri L, Donelli G. HT-29 cells: a new substrate for rotavirus growth. Arch Virol. 1991; 116:159–173. PMID: 1848062.

6. Hu W, Zhang H, Han Q, et al. A Vero-cell-adapted vaccine donor strain of influenza A virus generated by serial passages. Vaccine. 2015; 33:374–381. PMID: 25448099.

7. Blaney JE Jr, Manipon GG, Firestone CY, et al. Mutations which enhance the replication of dengue virus type 4 and an antigenic chimeric dengue virus type 2/4 vaccine candidate in Vero cells. Vaccine. 2003; 21:4317–4327. PMID: 14505914.

8. Zeiger RS. Current issues with influenza vaccination in egg allergy. J Allergy Clin Immunol. 2002; 110:834–840. PMID: 12464947.

9. World Health Organization. Japanese encephalitis [Internet]. Geneva: World Health Organization;2014. cited 2018 Jul 1. Available from: http://www.who.int/biologicals/vaccines/japanese_encephalitis/en/.
10. Ramani S, Crawford SE, Blutt SE, Estes MK. Human organoid cultures: transformative new tools for human virus studies. Curr Opin Virol. 2018; 29:79–86. PMID: 29656244.

11. Dutta D, Heo I, Clevers H. Disease modeling in stem cell-derived 3D organoid systems. Trends Mol Med. 2017; 23:393–410. PMID: 28341301.

12. Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009; 459:262–265. PMID: 19329995.

13. Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011; 470:105–109. PMID: 21151107.

14. Finkbeiner SR, Zeng XL, Utama B, Atmar RL, Shroyer NF, Estes MK. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. MBio. 2012; 3:e00159–e00112. PMID: 22761392.

15. Ettayebi K, Crawford SE, Murakami K, et al. Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016; 353:1387–1393. PMID: 27562956.

16. Garcez PP, Loiola EC, Madeiro da, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016; 352:816–818. PMID: 27064148.

17. Cugola FR, Fernandes IR, Russo FB, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016; 534:267–271. PMID: 27279226.

18. Chen YW, Huang SX, de Carvalho A, et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol. 2017; 19:542–549. PMID: 28436965.

19. Sainz B Jr, TenCate V, Uprichard SL. Three-dimensional Huh7 cell culture system for the study of Hepatitis C virus infection. Virol J. 2009; 6:103. PMID: 19604376.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download