3. Gordon SV, Parish T. Microbe profile: Mycobacterium tuberculosis: humanity's deadly microbial foe. Microbiology. 2018; 164:437–439. PMID:
29465344.

4. van Soolingen D, Hoogenboezem T, de Haas PE, et al. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, Canetti: characterization of an exceptional isolate from Africa. Int J Syst Bacteriol. 1997; 47:1236–1245. PMID:
9336935.

5. Niemann S, Rusch-Gerdes S, Joloba ML, et al. Mycobacterium africanum subtype II is associated with two distinct genotypes and is a major cause of human tuberculosis in Kampala, Uganda. J Clin Microbiol. 2002; 40:3398–3405. PMID:
12202584.
6. Niobe-Eyangoh SN, Kuaban C, Sorlin P, et al. Genetic biodiversity of Mycobacterium tuberculosis complex strains from patients with pulmonary tuberculosis in Cameroon. J Clin Microbiol. 2003; 41:2547–2553. PMID:
12791879.
7. Pfyffer GE, Auckenthaler R, van Embden JD, van Soolingen D. Mycobacterium canettii, the smooth variant of M. tuberculosis, isolated from a Swiss patient exposed in Africa. Emerg Infect Dis. 1998; 4:631–634. PMID:
9866740.

8. Panteix G, Gutierrez MC, Boschiroli ML, et al. Pulmonary tuberculosis due to Mycobacterium microti: a study of six recent cases in France. J Med Microbiol. 2010; 59:984–989. PMID:
20488936.

9. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997; 156(2 Pt 2):S1–S25. PMID:
9279284.
10. Kwon YS, Koh WJ. Diagnosis and treatment of nontuberculous Mycobacterial lung disease. J Korean Med Sci. 2016; 31:649–659. PMID:
27134484.

11. Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci. 2005; 20:913–925. PMID:
16361797.

12. Koh WJ, Kwon OJ, Jeon K, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006; 129:341–348. PMID:
16478850.

13. Park YS, Lee CH, Lee SM, et al. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis. 2010; 14:1069–1071. PMID:
20626955.
14. Escalante P. In the clinic: tuberculosis. Ann Intern Med. 2009; 150:ITC61–ITC614. PMID:
19487708.
15. Metcalfe JZ, Everett CK, Steingart KR, et al. Interferon-gamma release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. 2011; 204(Suppl 4):S1120–S1129. PMID:
21996694.
17. Sester M, Sotgiu G, Lange C, et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011; 37:100–111. PMID:
20847080.
18. Darban-Sarokhalil D, Imani Fooladi AA, Maleknejad P, et al. Comparison of smear microscopy, culture, and real-time PCR for quantitative detection of Mycobacterium tuberculosis in clinical respiratory specimens. Scand J Infect Dis. 2013; 45:250–255. PMID:
23113553.

19. Huh HJ, Kwon HJ, Ki CS, Lee NY. Comparison of the genedia MTB detection kit and the cobas TaqMan MTB assay for detection of Mycobacterium tuberculosis in respiratory specimens. J Clin Microbiol. 2015; 53:1012–1014. PMID:
25568443.

20. Perng CL, Chen HY, Chiueh TS, Wang WY, Huang CT, Sun JR. Identification of non-tuberculous mycobacteria by real-time PCR coupled with a high-resolution melting system. J Med Microbiol. 2012; 61:944–951. PMID:
22493281.

21. Centers for Disease Control and Prevention (CDC). Availability of an assay for detecting Mycobacterium tuberculosis, including rifampin-resistant strains, and considerations for its use, United States, 2013. MMWR Morb Mortal Wkly Rep. 2013; 62:821–827. PMID:
24141407.
22. Yang HY, Lee HJ, Park SY, Lee KK, Suh JT. Comparison of In-house polymerase chain reaction assay with conventional techniques for the detection of Mycobacterium tuberculosis. Korean J Lab Med. 2006; 26:174–178. PMID:
18156721.

23. Park CM, Heo SR, Park KU, et al. Isolation of nontuberculous mycobacteria using polymerase chain reaction-restriction fragment length polymorphism. Korean J Lab Med. 2006; 26:161–167. PMID:
18156719.

24. Nagy A, Vitaskova E, Cernikova L, et al. Evaluation of TaqMan qPCR system integrating two identically labelled hydrolysis probes in single assay. Sci Rep. 2017; 7:41392. PMID:
28120891.

25. Armstrong PM, Prince N, Andreadis TG. Development of a multi-target TaqMan assay to detect eastern equine encephalitis virus variants in mosquitoes. Vector Borne Zoonotic Dis. 2012; 12:872–876. PMID:
22835151.

26. Wang HY, Jin H, Bang H, et al. Evaluation of MolecuTech Real MTB-ID for MTB/NTM detection using direct specimens. Korean J Clin Microbiol. 2011; 14:103–109.

27. Baek YK. The evaluation of tuberculosis examination kit using a real-time PCR [thesis]. Cheongju: Chungbuk National University Graduate School of Biology;2014.
28. Park KS, Kim JY, Lee JW, et al. Comparison of the Xpert MTB/RIF and Cobas TaqMan MTB assays for detection of Mycobacterium tuberculosis in respiratory specimens. J Clin Microbiol. 2013; 51:3225–3227. PMID:
23863563.

29. Yang YC, Lu PL, Huang SC, Jenh YS, Jou R, Chang TC. Evaluation of the Cobas TaqMan MTB test for direct detection of Mycobacterium tuberculosis complex in respiratory specimens. J Clin Microbiol. 2011; 49:797–801. PMID:
21177901.

30. Lim J, Kim J, Kim JW, et al. Multicenter evaluation of Seegene Anyplex TB PCR for the detection of Mycobacterium tuberculosis in respiratory specimens. J Microbiol Biotechnol. 2014; 24:1004–1007. PMID:
24786527.

31. Lee MR, Chung KP, Wang HC, et al. Evaluation of the Cobas TaqMan MTB real-time PCR assay for direct detection of Mycobacterium tuberculosis in respiratory specimens. J Med Microbiol. 2013; 62:1160–1164. PMID:
23657531.

32. Choe W, Kim E, Park SY, Chae JD. Performance evaluation of Anyplex plus MTB/NTM and AdvanSure TB/NTM for the detection of Mycobacterium tuberculosis and nontuberculous mycobacteria. Ann Clin Microbiol. 2015; 18:44–51.
33. Lee JH, Kim BH, Lee MK. Performance evaluation of Anyplex Plus MTB/NTM and MDR-TB detection kit for detection of mycobacteria and for anti-tuberculosis drug susceptibility test. Ann Clin Microbiol. 2014; 17:115–122.

34. Ahn YO. Concepts and necessity of preventive medical services for the 21st century. J Korean Med Assoc. 2011; 54:246–249.

36. Nguipdop-Djomo P, Heldal E, Rodrigues LC, Abubakar I, Mangtani P. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: a retrospective population-based cohort study. Lancet Infect Dis. 2016; 16:219–226. PMID:
26603173.

37. Orme IM. Tuberculosis vaccine types and timings. Clin Vaccine Immunol. 2015; 22:249–257. PMID:
25540272.

38. Kaufmann SH, Fortune S, Pepponi I, Ruhwald M, Schrager LK, Ottenhoff TH. TB biomarkers, TB correlates and human challenge models: New tools for improving assessment of new TB vaccines. Tuberculosis (Edinb). 2016; 99(Suppl 1):S8–S11. PMID:
27402312.

39. Bedwell J, Kairo SK, Behr MA, Bygraves JA. Identification of substrains of BCG vaccine using multiplex PCR. Vaccine. 2001; 19:2146–2151. PMID:
11228387.

40. Minassian AM, Satti I, Poulton ID, Meyer J, Hill AV, McShane H. A human challenge model for Mycobacterium tuberculosis using Mycobacterium bovis bacille Calmette-Guerin. J Infect Dis. 2012; 205:1035–1042. PMID:
22396610.
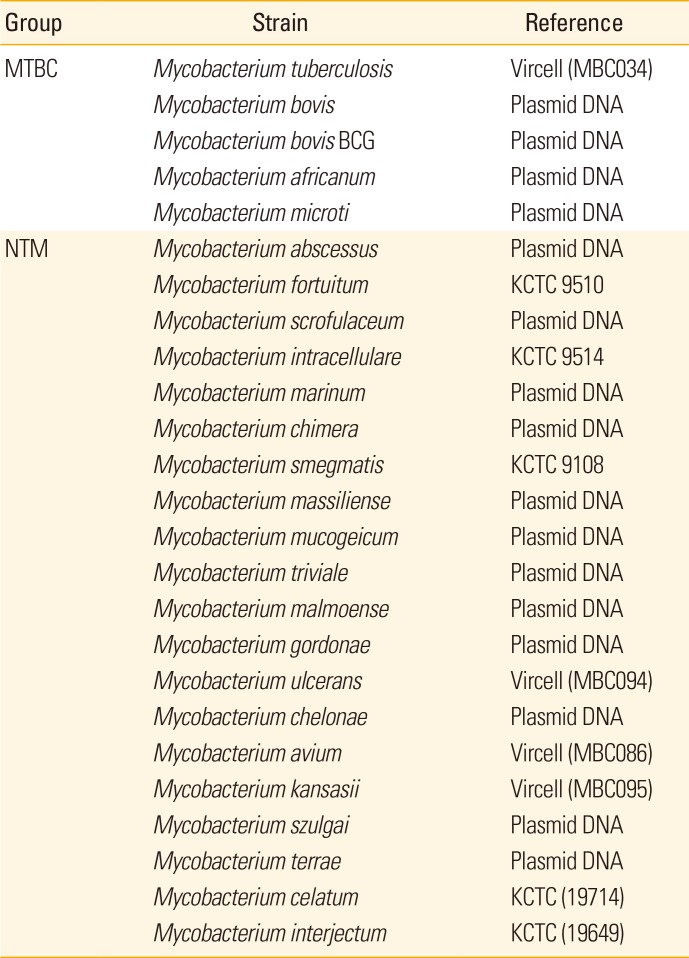
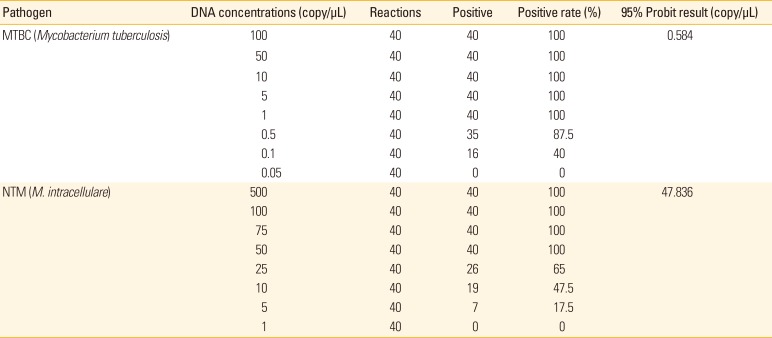
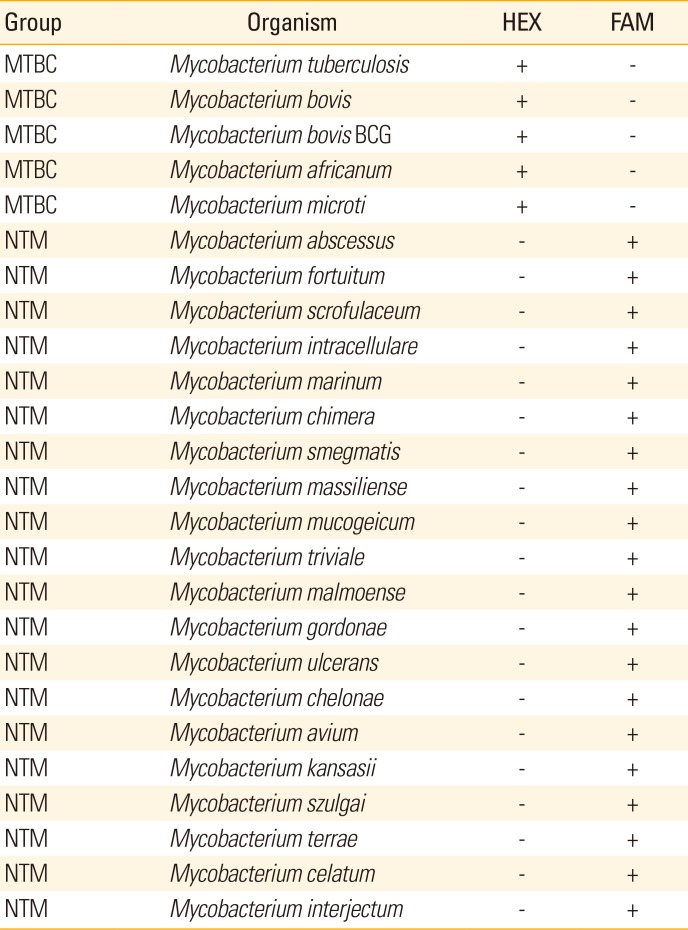
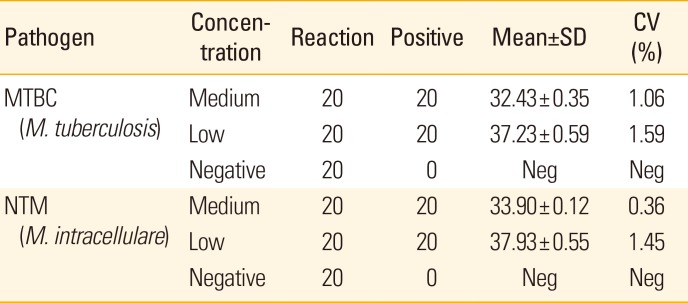
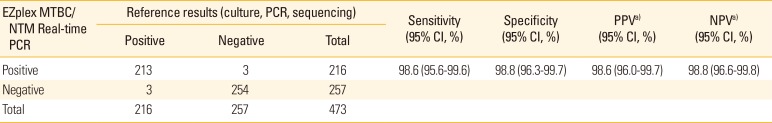
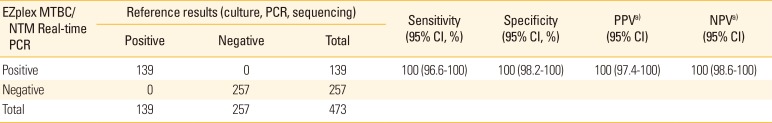




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download