Abstract
Pediatric breast disease is uncommon, and primary breast carcinoma in children is extremely rare. Therefore, the approach used to address breast lesions in pediatric patients differs from that in adults in many ways. Knowledge of the normal imaging features at various stages of development and the characteristics of breast disease in the pediatric population can help the radiologist to make confident diagnoses and manage patients appropriately. Most breast diseases in children are benign or associated with breast development, suggesting a need for conservative treatment. Interventional procedures might affect the developing breast and are only indicated in a limited number of cases. Histologic examination should be performed in pediatric patients, taking into account the size of the lesion and clinical history together with the imaging findings. A core needle biopsy is useful for accurate diagnosis and avoidance of irreparable damage in pediatric patients. Biopsy should be considered in the event of abnormal imaging findings, such as non-circumscribed margins, complex solid and cystic components, posterior acoustic shadowing, size above 3 cm, or an increase in mass size. A clinical history that includes a risk factor for malignancy, such as prior chest irradiation, known concurrent cancer not involving the breast, or family history of breast cancer, should prompt consideration of biopsy even if the lesion has a probably benign appearance on ultrasonography.
Breast lesions in children and adolescents are rare and different from adult breast disease in several respects. First, breast disease in children and adolescents includes mainly benign lesions related to normal development of the breast and benign tumors. Second, malignancies in children and adolescents are very rare (12345). Breast cancers in adolescents account for 0.1% of all breast cancers and less than 1% of all pediatric cancers (1236). Third, the clinical imaging approaches used for management of breast lesions in children and adolescents differ from those used for early detection of breast cancer in adults, given that children and adolescents rarely have malignant lesions (12378). Given these differences, management of breast disease in children and adolescents should be different from that in adults.
Most pediatric breast lesions are managed conservatively. Interventional treatment may affect developing breast buds in children and adolescents, so should be recommended cautiously based on clinical and imaging features (127).
Radiologists should be aware of the characteristics of pediatric breast disease and recognize the differences between children and adults for the purposes of appropriate evaluation and management. In this article, we review the differential diagnosis of breast lesions in children and adolescents, including development of benign and malignant disease, and make recommendations for their management.
An imaging evaluation of the adult breast is performed for early diagnosis of breast cancer. Mammography can diagnose malignant disease with microcalcifications; hence, it is the first imaging modality. However, mammography is not generally performed in the pediatric population because the mammary glands in developing adolescents are highly sensitive to ionizing radiation and adolescents have dense breasts with profuse fibroglandular tissue. Therefore, ultrasonography (US) is the preferred method because it can detect lesions in dense breast tissue and does not expose pediatric patients to ionizing radiation (1278). Psychological issues should be kept in mind when performing breast US in girls and teenagers, who may react sensitively to breast examinations, so require appropriate screening with provision of reassurance and comfort. US examinations can be performed using a high-resolution 15–17-MHz linear probe. A useful landmark for defining the posterior boundary of the breast is the pectoralis muscle (13). Radiologists should know the pitfalls associated with the normal anatomic structures seen on US. A rib or nipple may be mistaken for an abnormal lesion. On a cross-sectional scan, the cartilaginous portion of a rib can mimic a breast mass. A rib located posteriorly in the pectoralis muscle shows strong posterior acoustic shadowing, appearing as an elongated lesion in the longitudinal scan. Occasionally, patients complain of a prominent costochondral junction as a palpable mass (Fig. 1). Cooper's ligaments, which are normal structures, show posterior shadowing. Any of these structures might be misinterpreted as an abnormality. It is possible to identify such findings on US as normal by removing the posterior shadowing via adjustment of the angle of the transducer and controlling the pressing pressure (18). The nipple also creates a strong posterior acoustic shadow and can be misinterpreted as a subareolar mass. Appropriate compression and angulation of the transducer can eliminate posterior acoustic shadowing, allowing an anatomic structure to be easily recognized as normal on real-time US. A fat lobule is occasionally seen as an isoechoic solid mass, especially in the breast parenchyma. However, the fat lobule can be seen as a normal structure by rotating the transducer and confirming its integration with the surrounding normal fat tissue (9).
When CT scans are used for examination of thoracic disease in pediatric patients, breast lesions could be found incidentally (Fig. 2) (12). Magnetic resonance imaging (MRI) of the breast is not widely used in pediatric patients, but can facilitate surgical planning and identification of vessel or lymphatic anomalies at various anatomic sites (Fig. 3A, B) (128). As in adults, breast masses in pediatric patients can be assessed morphologically and hemodynamically using breast MRI.
The female breast undergoes two stages of development. The first stage starts at weeks 5–6 of fetal gestation. Epidermal cells invaginate and the primary mammary ridge starts to grow from both axillae to the inguinal region. Involution of milk lines occurs except at the level of the fourth intercostal space where the normal breast buds form. The remaining breast buds at the fourth intercostal space evolve into secondary buds and then into branching lactiferous ducts in the parenchyma of the breast. A small mammary pit forms on the skin surface overlying the breast buds and develops further into a nipple-areolar complex (12378). Bilateral subareolar nodules are common in neonates. These are temporary manifestations of physiologic wedge-shaped development in response to maternal hormones and disappear within 12 months (Fig. 3C). The breast buds are often asymmetric in size and show a clustered appearance in the first developmental stage. Unilateral breast development may have the appearance of a subareolar breast lump (1237810).
The second developmental stage occurs during adolescence and is known as thelarche. Hormones affect the development of breast buds in adolescent girls. Estrogen hormones are involved in elongation and differentiation of the ducts while progesterone hormones promote development of the terminal lobules. The mean age of onset of thelarche is 9.8 years. Premature thelarche is defined as onset before 8 years of age and delayed thelarche is defined as onset after 13 years of age (Fig. 4) (123811). Early development of the breast may occur alone or in association with precocious puberty. Idiopathic premature thelarche may occur in girls aged 1–3 years; this is not associated with precocious puberty and usually regresses, so it is sufficient to reassure the patient and caregiver (Fig. 5). When hypoechoic subareolar breast buds are identified on US without other signs of precocious puberty, clinical follow-up is sufficient and no further imaging or intervention is needed (1238). However, if early breast development is associated with symptoms of secondary sexual maturation, further evaluations, including measurement of bone age and abdominal and pelvic US, are needed to assess the development of the uterus and ovaries and to exclude an adrenal gland tumor (1238) (Fig. 6).
The developmental stages of adolescence are based on the Tanner scale. Breast development is divided into five clinical stages. Breast US can be used to obtain images reflecting the five stages of the Tanner scale. Findings on US may overlap between Tanner stages 2 and 4. However, there is a correlation between estrogen hormones and evaluation of development of breast tissue using US (1238). A US comparison of Tanner stages 1–5 is shown in Figure 7 (123812).
An accessory nipple (polythelia) and an accessory breast (polymastia) are congenital anomalies associated with incomplete regression of the mammary ridge. These anomalies can occur anywhere along the embryonic mammary ridge from the axilla to the groin, but most commonly occur in the axilla or inframammary fold (23). An accessory nipple is found in 1–2% of the population and can be clinically misdiagnosed as a nevus or pigmentation (123713). Clinically, an accessory breast in the axilla usually manifests as periodic pain with a protruded axilla. US can confirm the presence of fibroglandular tissue in the axilla (Fig. 8) (12378). Hypoplasia and amastia (absence of breast tissue, nipple, and areola) are rare and occur in Poland syndrome (Fig. 9). Amazia differs from amastia in that the nipple and areola are present but the underlying breast tissue is absent; it may be attributable to iatrogenic resection of the breast buds or radiation therapy before adolescence (121415).
Gynecomastia refers to excessive development of breast tissue in male individuals, including neonates, adolescents, and elderly men (16). About 60–75% of cases occur in adolescent boys (3). Clinically, patients with gynecomastia complain of a palpable mass or tenderness in the subareolar region of the breast. Gynecomastia appears as unilateral or asymmetric development of the breast tissue. It is thought to be triggered by an imbalance in estrogen and testosterone levels. Leptin, an enzyme in fat tissue, plays a role in increasing estrogen levels and leads to the development of breast tissue in male individuals. Drugs such as anabolic steroids, antidepressants, and antibiotics may also induce gynecomastia (1238). It is important to reassure patients and their caregivers that gynecomastia in newborns and adolescents usually disappears within two years. However, excessive and persistent gynecomastia may require further evaluation for tumors such as Sertoli-Leydig testicular, adrenocortical, or hepatoblastoma that can produce estrogen hormones. The possibility of liver disease or Klinefelter syndrome should also be considered (1278). When there is a palpable subareolar breast mass, US can identify increased subareolar fibroglandular tissue and exclude tumors (Fig. 10). Occasionally, obese patients may develop pseudogynecomastia, which is characterized by fat without fibroglandular tissue (13).
Simple cysts in the adult breast are commonly found at the age of 35–50 years but can appear at any age. These lesions fall within the spectrum of fibrocystic disease and may arise from dilated lobular acini because of an imbalance between fluid secretion and absorption or duct obstruction (17). A cyst in adolescence appears as an asymptomatic mass adjacent to the nipple, and in some patients may be accompanied by inflammation or symptoms such as lactation (2318). On US, a cyst appears as a round or oval anechoic mass with posterior acoustic enhancement and lacks blood flow on a color Doppler study. Simple asymptomatic cysts with typical imaging features do not require special treatment, further examination, or intervention. However, cysts with atypical imaging features, such as internal echogenicity, fluid-fluid level, internal septations, or a thick wall, may require aspiration to differentiate from other possible diagnoses, such as galactocele, abscess, or complicated cyst (148).
Duct ectasia is a rare entity that occurs in newborns and young children. Clinically, patients with duct ectasia present with bloody discharge from the nipple or a palpable mass in the breast. On US, multiseptate cyst-like masses or tubular anechoic structures are seen in the subareolar regions (Fig. 11). The etiology of duct ectasia is unknown, but maternal hormones might play an important role. Conservative treatment is indicated in newborns with these findings. Breast-feeding should be stopped and antibiotics should be used when necessary (128).
Mastitis and abscess are common in lactating women. However, they can also occur in pediatric cases with a bimodal distribution that includes infants (aged < 2 months) and older children (aged 8–17 years) (5). Mastitis is a clinical diagnosis based on signs and symptoms of infection, i.e., fever, erythema, and tenderness. Staphylococcus or pneumococci are the most common causative organisms in adolescence (1478). US is useful when performing fine-needle aspiration for diagnostic and therapeutic purposes. On US, an abscess appears as a complex cyst with a wall of variable thickness and is surrounded by increased blood flow on color Doppler study (Fig. 12) (123478). Treatment of mastitis with abscess includes antibiotic therapy and drainage with fine-needle aspiration. A large abscess may require surgical incision and drainage with follow-up examination to confirm resolution (12378).
Galactocele is a retention cyst filled with milk caused by obstruction of a lactiferous duct. It is commonly seen in pregnant or lactating women. However, galactoceles can also be seen in infants and adolescents. On US, galactoceles show various imaging features depending on the relative composition of fat and water (anechoic cysts to complex cysts). US showing a fat-fluid level within the cyst is diagnostic of galactocele (1278). Uncomplicated galactoceles with no atypical imaging features are self-limiting and do not need further evaluation or treatment. As with simple cysts, US-guided fine-needle aspiration should be performed in patients with symptoms and those with atypical imaging features (12719).
Hematoma or fat necrosis may be associated with a history of breast trauma or surgery. A recent history of trauma or surgery is essential for the diagnosis (1). US of the hematoma reveals changes over time depending on the degree of liquefaction. A hematoma appears as a hyperechoic focal lesion with an ill-defined margin in the acute phase and then changes into a cystic lesion with internal debris and septations (128). On US, fat necrosis is typically located in the subcutaneous fat layer, showing a variety of echo patterns ranging from solid complex cysts to oil cysts depending on the age of the lesion (20). Short-term imaging follow-up is recommended in patients with a typical clinical history and typical imaging features. Atypical imaging features or growth over time are indications for aspiration for both diagnostic and therapeutic purposes (12).
Fibroadenomas are the most common benign tumors in children and adolescents, accounting for 54–94% of cases. The fibroepithelial tissue of an adenoma is a benign proliferation that is clinically palpable (1245). Fibroadenomas are sensitive to estrogen and grow rapidly during pregnancy and puberty (21). Fibroadenoma is classified according to its size and histologic characteristics. The most common type is conventional fibroadenoma, which is usually 2–3 cm in size (22). If a fibroadenoma is larger than 5–10 cm, it is called a giant fibroadenoma (Fig. 13). Juvenile (cellular) fibroadenoma is a relatively rare variant of fibrous adenoma with hypercellular stromal proliferation and rapid growth and accounts for 7–8% of cases (1258). US findings for a typical fibroadenoma include a hypoechoic mass with a round or oval shape, a circumscribed margin, and orientation parallel to the skin (Fig. 14). Posterior acoustic enhancement is frequently seen and altered blood flow can be observed on color Doppler US (13458).
Follow-up US can be performed for small-sized fibroadenomas less than 3 cm in size that have a typical appearance on imaging and symptoms. During the first year, US can be performed every 6 months with a follow-up US examination after a further year to confirm the stability of the lesion over two years. Occasionally, fibroadenomas show atypical features on US, such as an angular border or posterior acoustic shadowing (58). If there are suspicious imaging features with rapid growth or symptoms, biopsy or surgical resection should be considered (125823).
Differential diagnoses include phyllodes tumors or pseudoangiomatous stromal hyperplasia (PASH) that are hard to distinguish on US because of their shared imaging features (1257).
PASH is a rare disease in children and is characterized by proliferation of hormone-mediated mesenchymal cells present in the background of breast tissue and associated with other breast pathologies. PASH has been seen in pre-menopausal women, but is rarely reported in children and adolescents. PASH has been reported to be associated with neurofibromatosis type 1 and immunodeficiency status. When PASH forms a tumor, its clinical and US findings are similar to those of fibroadenoma (Fig. 15). Follow-up US is sufficient, although surgery is indicated for large-sized lesions (127).
Juvenile papillomatosis is a very rare benign disease. It is also referred to as Swiss cheese disease and is characterized by small fibrous masses with localized multiple cysts and dilated ducts (724). It occurs in late adolescence at an average age of 19 years and may be associated with underlying benign breast tumors. Clinically, it is a hard and movable tumor that cannot be distinguished from fibroadenoma. US can facilitate the diagnosis of juvenile papillomatosis by revealing multiple peripheral small cysts with ill-defined margins (Fig. 16). Associated clustered microcalcifications can be seen (2). Juvenile papillomatosis is a histologically benign lesion, but is associated with an increased risk of breast cancer. Approximately 5–15% of patients have breast cancer and juvenile papillomatosis concomitantly, so continuous monitoring is warranted. A family history of breast cancer, histopathologic findings of atypia, bilaterality, multiplicity of lesions, and recurrence are associated with a high risk of malignancy. Treatment involves complete resection, because recurrence may occur if the lesion is partially resected (127825).
Phyllodes tumor is a rare form of fibroepithelial neoplasm that requires differentiation from fibroadenoma. Histologically, phyllodes tumors are classified into low, intermediate, and high grade. All histologic types can recur but rarely metastasize. There is a very rare malignant subtype of phyllodes tumor that has the features of a low-grade spindle cell sarcoma (13482627) and is common in women in their thirties. It accounts for less than 1% of pediatric breast diseases, but is the most common primary malignant breast tumor of adolescence (13626). Although 85% of phyllodes tumors in children and adolescents are benign, cases of infiltration, metastasis, or recurrence have been reported, with a mortality rate of about 3% (136). US findings for phyllodes tumors are not distinguishable from those for fibroadenomas. Typical phyllodes tumors are circumscribed, oval, and hypoechoic solid masses on US (Fig. 17). A peripheral cystic component and cleft may be seen more often in phyllodes tumors than in fibroadenomas but are not unique to phyllodes tumors (1234826) (Fig. 18).
The US features of a phyllodes tumor are similar to those of a fibroadenoma, and a core needle biopsy could be used to distinguish between this tumor and fibroadenoma (81023). If the tumor has enlarged rapidly or cysts are seen within the tumor, a US-guided core needle biopsy is warranted (12428). If the size is greater than 5 cm, surgical resection is indicated, because the ability of a core needle biopsy to distinguish between fibroadenoma and phyllodes tumor is limited (127823). If the tumor is diagnosed as a phyllodes tumor on biopsy, wide surgical excision with a safety margin of 1–2 cm should be performed regardless of histologic subtype. The prognosis is generally good. However, recurrence occurs even after complete resection in at least 20% of benign phyllodes tumors (726). Metastasis of phyllodes tumor is rare but may occur via hematogenous spread. The lung is the usual site of metastasis (7).
Malignant breast masses are quite rare in pediatric populations. Metastatic disease or hematologic malignancy is a more common etiology of a malignant breast mass than breast carcinoma in children and adolescents. A malignant breast mass may occasionally occur in a pediatric patient as a result of hematologic malignancy, usually lymphoma/leukemia, or metastasis from rhabdomyosarcoma or neuroblastoma. Malignant phyllodes tumor is the most common primary breast malignancy in pediatric populations (123567823).
Metastasis to the breast indicates dissemination of disease with a poor prognosis. Although multiple and bilateral tumors are suggestive of metastatic malignancy, benign fibroadenomas can also appear as multifocal and bilateral lesions. However, multifocal or bilateral lesions are not characteristic features of a malignant breast lesion (8). Breast metastases are seen as irregular, inhomogeneous, and hypoechoic masses on US. They may also appear in various forms. Breast metastases in patients with leukemia or lymphoma can be seen as circumscribed and hypoechoic solid masses (229). MRI is helpful in identifying multiple lesions or extensive disease. Although variable MRI features of lymphoma/leukemia have been observed, lymphoma can be seen as high-signal intensity on T2-weighted images with rim or heterogeneous enhancement and rapid enhancement kinetics on dynamic enhancement images (Fig. 19) (22930). It is important to be aware that metastatic cancers of the breast have variable imaging features that often suggest the lesion is probably benign. Therefore, a patient with known underlying primary extramammary malignancy who presents with a breast lesion should be carefully considered for breast biopsy even if the imaging features suggest that the lesion is probably benign (12).
Primary breast cancer is extremely rare in children and adolescents. The incidence of breast cancer is 1 in 1000000 in women younger than 20 years, but is significantly higher in women older than 25 years (12631). The most common histologic subtype of primary breast cancer in childhood is secretory breast carcinoma, which has a relatively favorable prognosis (3233). Radiation therapy to the chest wall is a major risk factor. Furthermore, the risk of breast cancer has been reported to increase by 75-fold when a patient aged 10–16 years is treated with radiotherapy for Hodgkin's disease (78). Risk factors for breast cancer include gene mutations such as BRCA1 or BRCA2 (128). Primary breast cancers are irregular in shape with a non-circumscribed margin, non-parallel orientation, a heterogeneously hypoechogenicity, posterior shadowing, and increased blood flow on US (Fig. 20). Associated features, such as architectural distortion, ductal changes, and ipsilateral axillary lymphadenopathy, can also increase the suspicion of malignancy (7). Microcalcifications with fine pleomorphic or fine linear branching morphology and grouped or segmental distribution on mammography are highly suspicious findings. However, mammography is not routinely performed in children and has limited diagnostic value. The imaging features of breast cancer in patients with BRCA1 mutation are similar to those of benign lesions such as fibroadenoma, so it is important to consider both clinical and imaging features in their management (3435). Breast-conserving surgery is feasible, although there is no consensus. Given that 20–30% of cases have axillary metastases, sentinel lymph node sampling is advocated (178).
Breast diseases in children and adolescents differ from those in adults. Assessment of imaging findings based on the Breast Imaging Reporting and Data System (BI-RADS) (36) is not commonly used in pediatric patients (158) because its purpose is to categorize the disease according to the possibility of malignancy, which is extremely rare in the pediatric population. The majority of pediatric breast lesions may be categorized as 2 (benign) or 3 (probably benign) with a recommendation of follow-up imaging for a period of time. Fibroadenoma is the most common breast tumor in children and adolescents. It is usually seen as a probably benign tumor with BI-RADS category 3 on US and has a malignancy potential of less than 2%. If clinical and US findings are benign, reassurance of the patient and/or caregiver is all that is necessary. Conservative follow-up examinations such as US are recommended (5823). In general, follow-up US is performed at 6-monthly intervals in the first year and a follow-up US after 12-month at the end of the second year. In pediatric breast disease, the size and growth of the mass can also affect management (158102337).
Surgical excision is suggested for masses with benign US features that are larger than 5 cm or rapidly growing (58). Gordon et al. (37) have suggested excision of masses that increase in size by more than 20% in 6 months or are larger than 3 cm in order to distinguish them from a phyllodes tumor. However, invasive surgery may damage normal developing breast tissue, and atypical phyllodes tumors are rare in pediatric patients. Many phyllodes tumors are similar to fibroadenomas. Core needle biopsy rather than surgery is recommended (81023). Sanders et al. (23) have shown that 1% of phyllodes tumors measure 3 cm or less while 3.6% are 3–5 cm, suggesting that 3 cm can be used as a criterion for core biopsy. Excision is suggested for masses measuring more than 5 cm. Although most pediatric breast lesions are benign, malignant disease is possible. Histologic examination is considered based on clinical features, medical history, family history, and US findings (156). Biopsy or surgical intervention is indicated. Even if imaging features are not suspicious in a patient with a high-risk clinical history, such as a history of other malignancies, a family history of breast cancer, prior chest irradiation, or genetic mutations, biopsy should be carefully considered (Fig. 21) (138). Core needle biopsy has high accuracy in the pediatric population, especially in differentiation of fibroadenomas and phyllodes tumors. Surgical excision is indicated in the event of incomplete tissue sampling or diagnostic difficulty with needle biopsy (123). A few benign entities, including trauma-related lesions and infection, can manifest as suspicious imaging features, and a detailed review of the clinical history is important before prompt interventional procedures (Fig. 22).
Diagnosis and management of breast diseases in children and adolescents is different from that in adults. Most breast lesions in children and adolescents are associated with breast development or are benign tumors. Therefore, their management should be conservative. US is an essential first imaging modality in pediatric patients. Many benign tumors appear similar on US, and sonographic follow-up is often required. Malignant tumors in pediatric patients are very rare. Histologic examination should be recommended carefully by combining clinical history, mass size, growth rate, and US findings in pediatric patients. Core needle biopsy is a useful technique that can provide an accurate diagnosis without causing irreparable damage in pediatric patients.
Figures and Tables
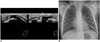 | Fig. 13-year-old girl with palpable lesion in left breast.
A. On ultrasonographic image, normal chostochondral junction is seen in scan of palpable area. Note that rib is located posterior to pectoralis muscle (arrow), showing posterior acoustic shadowing (arrowhead). No abnormality is noted in breast parenchyma. B. Plain chest radiograph showing cleavage of left fifth rib at costochondral junction, suggestive of bifid rib (arrow).
|
 | Fig. 215-year-old girl undergoing CT for evaluation of trauma.
A. CT scan reveals enhancing mass in left breast (arrow). B. Ultrasonographic image shows probably benign mass in left breast that correlates with CT findings. This patient was followed up without pathologic confirmation of probably benign mass. CT = computed tomography
|
 | Fig. 3Findings in 1-day-old boy who had palpable mass in right lateral portion of chest wall.
A. Ultrasonographic image revealed thin septate cyst suggesting lymphangioma. B. Fat suppressed T2-weighted MR image showing thin septate high-signal intensity lymphangioma. C. T1-weighted MR image showing proliferation of glandular tissue in subareolar area bilaterally and attributable to physiologically enlarged glandular tissue under influence of maternal hormones. MR = magnetic resonance
|
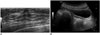 | Fig. 4Ultrasonographic images for 18-year-old girl with corpus callosum agenesis.
A. US of breast showing hypogenesis of fibroglandular tissue bilaterally. B. US of pelvis reveals immature uterus, suggestive of sexual immaturity. US = ultrasonography
|
 | Fig. 5Ultrasonographic image for 1-year-old girl who had palpable masses in both breasts.Image reveals proliferation of glandular tissue in subareolar area bilaterally with no other signs of precocious puberty. Clinical follow-up was sufficient and no further imaging or intervention was needed.
|
 | Fig. 6Ultrasonographic images for 9-year-old girl who had been diagnosed to have lump in each breast 6 months earlier.
A. Ultrasonographic image of breast showing hypoechoic linear projection of glandular tissue in subareolar area (Tanner stage 3). B. Ultrasonographic image of pelvis showing enlarged uterus with ovaries that had normal volume range (not shown).
|
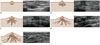 | Fig. 7Ultrasonographic comparison of Tanner stages.
A. Tanner stage 1. Clinical elevation of papilla is seen. US shows small foci echogenic tissue in subareolar area. B. Tanner stage 2. Clinical elevation of both breast and papilla is seen with small amount of enlargement in areolar diameter. US shows hypoechoic subareolar breast bud with hyperechoic breast parenchyma composed of adipose tissue and loose connective tissue. C. Tanner stage 3. Clinically palpable subareolar nodule with further enlargement of breast and areola are noted without separation of their contours. US shows extension of hyperechoic fibroglandular tissue with central spider-like and hypoechoic linear projections away from retroareolar region, reflecting elongated ducts. D. Tanner stage 4. Clinical projection of areola and papilla forms secondary mound above breast with separation of their contour. US shows more widely elongated hypoechoic breast bud and loss of rounded appearance. Subcutaneous fat may be present. E. Tanner stage 5. Clinical projection of papilla only with recession of areola to general contour of breast. US shows mature breast appearance, heterogeneous echogenicity of breast parenchyma intermixed with echogenic glandular and stromal tissue, and increased amount of subcutaneous fat.
|
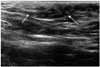 | Fig. 8Ultrasonographic image of breast for 12-year-old girl with lump in left axilla.Image shows focal area of heterogeneous fibroglandular tissue in subcutaneous fat layer of left axilla (arrows) similar to that of breast.
|
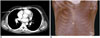 | Fig. 9Poland syndrome in 13-year-old boy.
(A) CT and (B) MIP reconstruction images showing absence of pectoralis muscle in right chest wall (arrow). MIP = maximum intensity projection
|
 | Fig. 10Gynecomastia in 18-year-old boy with palpable mass in subareolar region in both breasts.
A. Ultrasonographic image showing normal glandular breast tissue in subareolar regions of both breasts, indicating gynecomastia. B. Computed tomographic image showing linear or tubular soft tissue density in subareolar region of both breasts.
|
 | Fig. 11Duct ectasia in 3-day-old boy with lump in subareolar region bilaterally.
A, B. Ultrasonographic images showing heterogeneous multiseptate cystic mass-like lesion in subareolar region of both breasts as findings of duct ectasia.
|
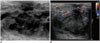 | Fig. 12Ultrasonographic images for 19-year-old girl with 2-week history of redness and pain in right breast.
A. Ultrasonographic image showing heterogeneous echoic mass-like lesion with diffuse fat infiltration in breast tissue, indicating abscess. B. Color Doppler study showing increased vascularity that suggests hypervascularity because of inflammation.
|
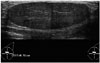 | Fig. 13Giant fibroadenoma in 15-year-old girl.Ultrasonographic image shows approximately 6.5-cm circumscribed, oval, parallel isoechoic mass in left breast.
|
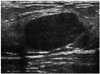 | Fig. 14Conventional fibroadenoma in 17-year-old girl.Ultrasonographic images shows circumscribed, oval, parallel, isoechoic mass with posterior enhancement in left breast.
|
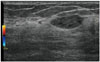 | Fig. 15PASH.Ultrasonographic image for 44-year-old woman shows oval, circumscribed, parallel, and hypoechoic mass with posterior acoustic enhancement in right breast. Imaging features are similar to those of fibroadenoma. US-guided core needle biopsy revealed PASH. PASH = pseudoangiomatous stromal hyperplasia
|
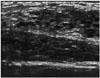 | Fig. 16Juvenile papillomatosis in 17-year old girl with nipple discharge.Ultrasonographic image shows Swiss cheese-like cystic duct ectasia in right breast. Surgical excision revealed intraductal papillomatosis.
|
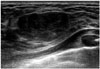 | Fig. 17Phyllodes tumor in 17-year-old girl with palpable mass in left breast.Ultrasonographic image shows 2.5-cm circumscribed, oval, isoechoic parallel mass in left breast. Vacuum-assisted biopsy revealed benign phyllodes tumor.
|
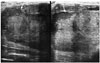 | Fig. 1814-year-old girl with palpable mass in breast.Ultrasonographic image shows right and central portions of huge mass. Huge, circumscribed, round mass with peripheral cystic component (arrows) can be seen. Patient was confirmed to have low-grade malignant phyllodes tumor.
|
 | Fig. 1917-year-old girl with non-Hodgkin's lymphoma.
A. Ultrasonographic showing multiple circumscribed, oval, hypoechoic masses with posterior enhancement in both breasts. B. Fat suppressed T2-weighted MR image showing multiple, round, inhomogeneous masses with high signal intensity in both breasts. C. Early subtraction T1-weighted MR image after administration of gadolinium contrast showing inhomogeneous enhancing breast masses bilaterally. Core needle biopsy revealed metastatic lymphoblastic B-cell lymphoma of breast.
|
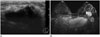 | Fig. 20Primary breast cancer in 23-year-old woman with family history of breast cancer.
A. Ultrasonographic image of breast showing irregular, indistinct, hypoechoic mass with posterior shadowing. B. MIP reconstruction-enhanced early subtraction MR image showing rapidly enhancing mass with neoangiogenetic vascular structures. Enlarged enhancing axillary lymph node is also noted in left axilla level I. US-guided core needle biopsy of left breast mass confirmed invasive ductal carcinoma.
|
 | Fig. 22Algorithm for sonographic assessment and management of pediatric breast lesions.
*BI-RADS assessment category 2: simple cyst. Oval, round, circumscribed, anechoic mass with posterior acoustic enhancement. **BI-RADS assessment category 3: oval, circumscribed, hypo-/iso-/hyperechogenic mass with various vascularity. ***BI-RADS assessment category 4 or 5: masses with suspicious features; irregular margin, nonparallel orientation, non-circumscribed margin, increased vascularity, heterogeneous echo pattern (including complex cystic and solid), associated features. BI-RADS = Breast Imaging Reporting and Data System, MRI = magnetic resonance imaging, Sx/sign = symptom/sign
|
References
1. Valeur NS, Rahbar H, Chapman T. Ultrasound of pediatric breast masses: what to do with lumps and bumps. Pediatr Radiol. 2015; 45:1584–1599. quiz 1581-1583.

2. Chung EM, Cube R, Hall GJ, González C, Stocker JT, Glassman LM. From the archives of the AFIP: breast masses in children and adolescents: radiologic-pathologic correlation. Radiographics. 2009; 29:907–931.
3. García CJ, Espinoza A, Dinamarca V, Navarro O, Daneman A, García H, et al. Breast US in children and adolescents. Radiographics. 2000; 20:1605–1612.

4. Kronemer KA, Rhee K, Siegel MJ, Sievert L, Hildebolt CF. Gray scale sonography of breast masses in adolescent girls. J Ultrasound Med. 2001; 20:491–496. quiz 498.

5. Sanchez R, Ladino-Torres MF, Bernat JA, Joe A, DiPietro MA. Breast fibroadenomas in the pediatric population: common and uncommon sonographic findings. Pediatr Radiol. 2010; 40:1681–1689.

6. Gutierrez JC, Housri N, Koniaris LG, Fischer AC, Sola JE. Malignant breast cancer in children: a review of 75 patients. J Surg Res. 2008; 147:182–188.

7. Kaneda HJ, Mack J, Kasales CJ, Schetter S. Pediatric and adolescent breast masses: a review of pathophysiology, imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2013; 200:W204–W212.

8. Gao Y, Saksena MA, Brachtel EF, terMeulen DC, Rafferty EA. How to approach breast lesions in children and adolescents. Eur J Radiol. 2015; 84:1350–1364.

9. Baker JA, Soo MS, Rosen EL. Artifacts and pitfalls in sonographic imaging of the breast. AJR Am J Roentgenol. 2001; 176:1261–1266.

10. Durmaz E, Öztek MA, Arıöz Habibi H, Kesimal U, Sindel HT. Breast diseases in children: the spectrum of radiologic findings in a cohort study. Diagn Interv Radiol. 2017; 23:407–413.

11. Bock K, Duda VF, Hadji P, Ramaswamy A, Schulz-Wendtland R, Klose KJ, et al. Pathologic breast conditions in childhood and adolescence: evaluation by sonographic diagnosis. J Ultrasound Med. 2005; 24:1347–1354. quiz 1356-1357.
12. Youn I, Park SH, Lim IS, Kim SJ. Ultrasound assessment of breast development: distinction between premature thelarche and precocious puberty. AJR Am J Roentgenol. 2015; 204:620–624.

13. Adler DD, Rebner M, Pennes DR. Accessory breast tissue in the axilla: mammographic appearance. Radiology. 1987; 163:709–711.

14. Mojallal A, La Marca S, Shipkov C, Sinna R, Braye F. Poland syndrome and breast tumor: a case report and review of the literature. Aesthet Surg J. 2012; 32:77–83.

15. Zhang F, Qi X, Xu Y, Zhou Y, Zhang Y, Fan L, et al. Breast cancer and Poland's syndrome: a case report and literature review. Breast J. 2011; 17:196–200.

17. Weinstein SP, Conant EF, Orel SG, Zuckerman JA, Bellah R. Spectrum of US findings in pediatric and adolescent patients with palpable breast masses. Radiographics. 2000; 20:1613–1621.

18. Huneeus A, Schilling A, Horvath E, Pinochet M, Carrasco O. Retroareolar cysts in the adolescent. J Pediatr Adolesc Gynecol. 2003; 16:45–49.

19. Welch ST, Babcock DS, Ballard ET. Sonography of pediatric male breast masses: gynecomastia and beyond. Pediatr Radiol. 2004; 34:952–957.

20. Soo MS, Kornguth PJ, Hertzberg BS. Fat necrosis in the breast: sonographic features. Radiology. 1998; 206:261–269.

21. Simmons PS. Diagnostic considerations in breast disorders of children and adolescents. Obstet Gynecol Clin North Am. 1992; 19:91–102.

22. Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: use of sonography to distinguish between benign and malignant lesions. Radiology. 1995; 196:123–134.

23. Sanders LM, Sharma P, El Madany M, King AB, Goodman KS, Sanders AE. Clinical breast concerns in low-risk pediatric patients: practice review with proposed recommendations. Pediatr Radiol. 2018; 48:186–195.

24. Patterson SK, Jorns JM. A case of juvenile papillomatosis, aka “Swiss cheese disease”. Breast J. 2013; 19:440–441.

27. Cecen E, Uysal KM, Harmancioglu O, Balci P, Kupelioglu A, Canda T. Phyllodes tumor of the breast in an adolescent girl. Pediatr Hematol Oncol. 2008; 25:79–82.

28. Yabuuchi H, Soeda H, Matsuo Y, Okafuji T, Eguchi T, Sakai S, et al. Phyllodes tumor of the breast: correlation between MR findings and histologic grade. Radiology. 2006; 241:702–709.

29. Surov A, Holzhausen HJ, Wienke A, Schmidt J, Thomssen C, Arnold D, et al. Primary and secondary breast lymphoma: prevalence, clinical signs and radiological features. Br J Radiol. 2012; 85:e195–e205.

30. Wienbeck S, Meyer HJ, Uhlig J, Herzog A, Nemat S, Teifke A, et al. Radiological imaging characteristics of intramammary hematological malignancies: results from a german multicenter study. Sci Rep. 2017; 7:7435.

31. Kennedy RD, Boughey JC. Management of pediatric and adolescent breast masses. Semin Plast Surg. 2013; 27:19–22.

32. Murphy JJ, Morzaria S, Gow KW, Magee JF. Breast cancer in a 6-year-old child. J Pediatr Surg. 2000; 35:765–767.

33. Horowitz DP, Sharma CS, Connolly E, Gidea-Addeo D, Deutsch I. Secretory carcinoma of the breast: results from the survival, epidemiology and end results database. Breast. 2012; 21:350–353.

34. Ha SM, Chae EY, Cha JH, Kim HH, Shin HJ, Choi WJ. Association of BRCA mutation types, imaging features, and pathologic findings in patients with breast cancer with BRCA1 and BRCA2 mutations. AJR Am J Roentgenol. 2017; 209:920–928.

35. Lee MV, Katabathina VS, Bowerson ML, Mityul MI, Shetty AS, Elsayes KM, et al. BRCA-associated cancers: role of imaging in screening, diagnosis, and management. Radiographics. 2017; 37:1005–1023.
36. Mendelson EB, Böhm-Vélez M, Berg WA, et al. ACR BI-RADS ultrasound. ACR BI-RADS Atlas. Breast imaging reporting and data system. 5th ed. Reston, VA: American College of Radiology;2013. p. 128–130.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download