Abstract
The aim of this systematic review was to describe the characteristics of prostate-specific membrane antigen (PSMA)-targeting PET and their clinical applications in prostate cancer patients. There have been major strides in the design, synthesis of PSMA-targeting PET tracers over the past several years. PSMA-targeting PET tracers can be categorized, according to positron emitters and targeting strategies for the PSMA. The majority of PSMA PET studies has been focused on patients with biochemical recurrence, but additional values of PSMA PET have also been investigated for use in primary staging, treatment planning, response evaluation, and PSMA radioligand therapy. PSMA PET is expected to bring improvements in the management of patients, but the impact of improved diagnosis by PSMA on overall survival remains unanswered. Many challenges still await PSMA PET to expedite the use in the clinical practice. At this early stage, prospective multicenter trials are needed to validate the effectiveness and usefulness of PSMA PET.
Prostate cancer (PCa) is one of the most common male cancers and is the leading cause of death in men worldwide (1). Approximately 15–40% of patients will experience a detectable rise in the serum level of prostate specific antigen (PSA) within 10 years after primary treatment, despite highly successful treatment results for localized PCa (2). Localized PCa can range from those with a low malignant potential to those with locally or systemically recurrence after successful local therapy. The latter category is broadly considered as ‘high-risk’ or alternatively ‘locally advanced’ in clinical practice. Additionally, many patients who die from PCa initially present with tumors seemingly confined to the prostate gland.
Prostate cancer is highly heterogeneous; therefore, risk stratification is often inappropriate to determine treatment managements and predict prognosis. PCa is currently classified into three risk groups (low, intermediate, and high) based on serum PSA level, Gleason score, and clinical stage (3). Incorrect risk stratification can be inherent with the biopsy method and tumor heterogeneity. It is possible that false-negative biopsies underrepresent high-grade tumor foci that are related to the tumor biology and clinical outcome. In practice, a standard 10- to 12-core biopsy could miss 38% of multifocal PCa (4), and this figure is not greatly improved by increasing the number of samples (5). High false-negative rates primarily stem from the heterogeneity of PCa, which is considered a major limitation of the Gleason grading system.
Imaging plays an important role in the management of PCa patients. Imaging is essential to assess the clinical stage in primary staging, which aids selection of the optimal treatment strategy and provides information for the accurate prognosis. Imaging also plays a key role in the surveillance of PCa patients. It is particularly important to distinguish between local recurrence and distant metastasis when developing appropriate treatment strategies for PCa patients with biochemical recurrence (BCR). Although serum PSA is a tissue-specific and sensitive tumor marker, the PSA level itself does not provide information regarding the origin of the PSA. In this regard, the development of accurate imaging is required as an effective diagnostic tool to detect, localize, and characterize PCa.
The prostate-specific membrane antigen (PSMA) is a promising target for both diagnosis and treatment of PCa, since it has appropriate properties as a biomarker in PCa patients. The PSMA is a type II transmembrane glycoprotein that is primarily expressed in prostatic tissues (6). The PSMA is overexpressed in nearly all primary prostate tumors in addition to metastatic tumors (7) and PSMA expression further increases in de-differentiated, metastatic, or hormone-refractory disease (89). Moreover, PSMA expression level is an independent prognostic factor for the clinical outcome of PCa (10). In addition, it is advantageous that the PSMA is not appreciably released into the circulation, in contrast to other candidates including highly specific prostate-related markers: PSA, prostate secretory protein, and prostatic acid phosphatase, which are secretory proteins (1112). Currently, the PSMA is being investigated intensively, as it holds promise for extending its application from imaging to therapy using therapeutic radionuclides.
Herein, we review the characteristics of PSMA ligands for PET imaging and their clinical applications in PCa. We will briefly introduce PCa-targeted PET tracers with different targeting strategies, since they have also been actively investigated as a promising imaging tool for PCa. In addition, we will discuss the development of PSMA ligands labeled with therapeutic radionuclides, which is expected to expedite the progress of PSMA PET imaging and enable the effective treatment of patients with advanced PCa.
Currently, two PCa-targeted PET tracers have gained Food and Drug Administration (FDA) approval in the United States for the identification of recurrent PCa: 11C-choline and anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (18F-FACBC). Choline PET assesses choline metabolism, which is altered in prostate tumor cells (13) and has demonstrated high sensitivity in the detection and localization of PCa (1415). However, its specificity is limited to the detection of primary PCa, because choline transporter expression and choline transport rate also increased in benign prostatic hyperplasia (1516). In addition, there is another promising candidate for FDA approval among choline PET tracers. 18F-fluorocholine has yet to acquire FDA approval in the United States, but it is officially registered on the European Pharmacopoeia and qualified as a New Health Technology in Korea. Based on the established credibility, 18F-fluorocholine has been actively investigated in PCa patients.
18F-FACBC (fluciclovine or Axumin™) is a radiolabeled amino acid analog that is accumulated after preferential uptake by prostate tumor cells, because it does not undergo further metabolism in the cells (1718). Fluciclovine PET was found to be successful in the assessment of primary and metastatic PCa (1920). Fluciclovine PET effectively localized the source of increased PSA in patients with BCR. In a meta-analysis evaluating 6 articles and 251 patients with BCR, the pooled sensitivity and specificity of fluciclovine PET on a per-patient analysis were 87% and 66%, respectively (21). These PCa-targeted PET tracers are not specifically targeting to prostate tumor cells, but are worthy of attention in this review, because they are promising competitors as PCa-targeted PET tracers.
The PSMA has appropriate characteristics as an ideal target for the evaluation of PCa. PSMA expression in PCa has been demonstrated to be 100- to 1000-fold greater than that in normal tissues (6). Furthermore, PSMA expression may increase as tumor grade and castration resistance increases (89). The PSMA is not appreciably released into the circulation, because it is an integral membrane protein of the prostate (11). After binding to the PSMA, PSMA ligands are internalized and undergo endosomal recycling, leading to enhanced uptake and retention in tumor cells. Based on these characteristics of PSMA ligands, high image quality for diagnostic procedures is induced and a high local dose for therapeutic applications is guaranteed. PSMA-targeting strategies are classified into two methods using antibodies or ligands.
Prostate-specific membrane antigen-targeting monoclonal antibodies (mAbs) were investigated in the late 90's. 111In-capromab pendetide (ProstaScint®) was approved by the FDA for the evaluation of patients with biopsy-proven PCa with high-risk of pelvic lymph node (LN) metastasis. However, the overall diagnostic accuracy of ProstaScint® fell short, since the murine mAb used for ProstaScint® (7E11-C5.3) binds to an intracellular epitope of the PSMA (15), which may be accessible only in dead, dying, or apoptotic cells within tumors. Accordingly, the next generation anti-PSMA mAbs were designed to bind to the extracellular portion of PSMA to enhance diagnostic accuracy. Besides targeting affinity and specificity, drawbacks are still remained in the use of radiolabeled mAbs as an effective diagnostic tool. Their long biological half-life in the circulation and poor penetrating ability to the solid tumor result in high non-specific background-to-tumor noise and reduce diagnostic accuracy.
Immuno-PET has been introduced to overcome the limitations of conventional antibody imaging, via modification of antibodies and selection of appropriate positron emitters. The half-life of a positron emitter should be long enough to achieve optimal background-to-tumor ratios, and an antibody or antibody fragment should be sufficiently maintained during in vivo binding. In this regard, studies have investigated biologically-engineered single chain fragments or minibodies combined with the longer-lived positron emitters such as 89Zr and 64Cu for immuno-PET. The introduction of immuno-PET is also an attractive novel option for PSMA-targeting imaging. The humanized antibody J591 (huJ591) directly targets the extracellular domain of the PSMA, and the modified form of huJ591 labeled with 89Zr showed promising results in clinical trials (22). The minibody IAB2M, genetically engineered from huJ591, labeled with 89Zr demonstrated superior results for the detection of bone metastases, compared to conventional imaging modalities in clinical phase I trial (22).
The PSMA has a unique feature that forms a ligand-receptor complex with a substrate. Based on this feature, small molecules mimicking the endogenous substrate for the PSMA that are labeled with radionuclides have been developed for the diagnosis and treatment. The basic chemical structure of these PSMA ligands incorporates glutamate-urea-glutamate or glutamate-urea-lysine dimers, which are essential structural components required for binding to the catalytic domain of PSMA (23). The first generation of PSMA ligands was glutamate-urea amino acid heterodimeric inhibitors of the PSMA, which was initially developed for scintigraphy and/or SPECT (24). Thereafter, PSMA ligands linked with a chelator for 68Ga complexation were developed for PET imaging, as PET imaging is much advantageous over scintigraphic imaging in terms of image resolution and quantification. In addition to 68Ga-labeled PSMA ligands, 18F-labeled PSMA ligands are available for clinical use. These PSMA ligands radiolabeled with positron emitters are of utmost clinical interest for both the diagnosis and treatment of PCa and will be discussed as a main topic in this review.
Prostate-specific membrane antigen ligands that are labeled with 68Ga, a positron emitter, are promising and widely available in clinical practice. Among 68Ga-PSMA ligands, 68Ga labeled with Glu-NH-CO-NH-Lys-(Ahx) (68Ga-HBED-CC or 68Ga-PSMA-11) is a leading PET tracer that is the most widely used and actively investigated in clinical settings. 68Ga-PSMA-11 has a strong binding affinity for the PSMA and is efficiently internalized into prostate tumor cells (25). Several biodistribution studies of 68Ga-PSMA-11 well demonstrated cellular expressions of PSMA across the body; in the lacrimal and salivary glands, liver, spleen, kidneys, and some parts of the intestines (262728) (Fig. 1). The uptake of 68Ga-PSMA-11 in these tissues is considered physiological, and the expression level of PSMA are markedly below than that of prostate tumor cells (29). On the other hand, unbound form of 68Ga-PSMA-11 is excreted via the kidneys and urinary tract (30). Another 68Ga-PSMA ligands such as 68Ga-PSMA-617, and 68Ga-PSMA-I&T have demonstrated similar biodistribution and imaging properties to 68Ga-PSMA-11. Due to their similarities each other, 68Ga-PSMA-11, 68Ga-PSMA-617, and 68Ga-PSMA-I&T are collectively known as 68Ga-PSMA ligands. 68Ga-PSMA ligands are advantageous over anti-PSMA antibodies since they are “small molecules.” These ligands possess high receptor affinity for the PSMA as aforementioned; they have excellent tissue penetrating abilities, and then diffuse well into solid tumor lesions such as bone metastases of PCa.
Although 68Ga-PSMA ligands have prevailed in the studies for the development of PCa imaging, there appears to be growing interests in developing 18F-PSMA ligands. In general, 18F-based PET would offer advantages over 68Ga-based PET with respect to availability, amount of production, and image resolution (Fig. 1). The first-generation 18F-PSMA ligand was N-[N-[(S)-1,3-dicarboxyprophyl]carbamoyl]-4-[18F]-fluorobenzyl-L-cysteine (18F-DCFBC), and a first-in-human study of 18F-DCFBC was performed in 5 patients with metastatic PCa (31). In this study, 18F-DCFBC had favorable dosimetry and a good biodistribution profile and was able to detect putative sites of occult disease that were not defined by conventional imaging modalities. However, 18F-DCFBC had persistently high blood-pool activity and relatively low background-to-tumor ratios. To overcome these limitations, the second generation 18F-PSMA ligand, 2-(3-(1-carboxy-5-((6-[18F]fluoro-pyridine-3-carbonyl)amino)-pendtyl)-ureido)-pentanedioic acid (18F-DCFPyL) was clinically introduced (3233). A first-in-human study of 18F-DCFPyL was performed in 9 patients with metastatic PCa; this ligand also exhibited favorable dosimetry, biodistribution, and safety profiles (33). Most recently, 18F-PSMA-1007 was developed so as to improve the biodistribution profiles of the previously developed 18F-PSMA ligands. 18F-PSMA-1007 reduces urine clearance, which could potentially facilitate the evaluation of the prostatic bed (34).
As aforementioned, different types of PSMA-targeting PET tracers have been investigated for the diagnosis and treatment of PCa. Unmet clinical needs for the management of PCa are identical for all the PSMA-targeting PET tracers, but the majority of studies have been performed using 68Ga-PSMA ligands, in particular 68Ga-PSMA-11. Hereafter, the critical review of clinical applications mainly focuses on studies investigating with 68Ga-PSMA-11. The clinical applications of PSMA PET are classified as follows; 1) BCR, 2) primary staging, 3) treatment planning/response evaluation, 4) PSMA radioligand therapy (RLT), 5) comparisons with other PCa-targeted PET tracers, and 6) the implementation of the hybrid PET/MRI.
Biochemical recurrence refers to the clinical status of an increasing serum PSA level after curative intent of local treatments in PCa patients. Currently, BCR is defined as two consecutive values of PSA > 0.2 ng/mL in patients receiving radical prostatectomy (RP). BCR is a commonly encountered but very difficult clinical situation for clinicians, because both the early detection of treatment failure and exact localization of tumor recurrence are important in treating patients with BCR. Different therapeutic options are available for the treatment of BCR after RP, according to the disease extent; a locoregional disease vs. systemic disease. In the absence of systemic disease, salvage radiation therapy could be the first treatment option for patients with BCR. In addition, metastasis-directed therapies could enhance therapeutic outcomes and reduce unnecessary side effects when administered at lower PSA levels (35). In particular, it has been reported that the patient's prognosis was improved when the salvage therapy was initiated before the PSA level exceeds 0.5 ng/mL (36). Therefore, there is a rising demand for a reliable and accurate diagnostic tool to detect tumor recurrence and assess its extent in patients with BCR.
Prostate-specific membrane antigen PET can detect recurrence sites at lower PSA levels than conventional imaging modalities, as well as localize the origin of PSA increase, because it is important to distinguish local recurrence and systemic metastases in order to plan therapeutic approach. Although MRI has proven to be useful in the detection of locoregional recurrence of PCa, but PSA levels in the studies were generally higher than those in PSMA PET studies. Several retrospective studies using MRI demonstrated high diagnostic accuracies with sensitivities of 53–95% in the detection of BCR, but mean PSA levels ranged from 1.23 ng/mL to 2.18 ng/mL (373839). In a recent study comparing a combined technique of multiparametric MRI (mpMRI) with 18F-fluorocholine PET, average serum level of PSA was 1.1 ng/mL in group A and 1.9 ng/mL in group B, retrospectively (40). PSMA PET, whereas, can detect BCR at a very lower level of PSA. The tumor detection rates ranged wide from the 50.0% to 89.5%, according to the PSA levels set by retrospective studies (4142). An overall detection rate was 89.5% in a retrospective series study for 248 patients after RP, and detection rates for early BCR differed by PSA levels; 57.9% at PSA levels of 0.2–0.5 ng/mL, and 72.7% at PSA levels of 0.5–1.0 ng/mL, respectively (41). The detection rates of 68Ga-PSMA PET for tumor recurrence gradually increased according to the PSA levels (Fig. 2) (42). In a recent meta-analysis analyzing 16 articles including 1309 patients, the pooled detection rate was 58% at PSA levels of 0.2–1.0 ng/mL, which increased to 76% with PSA levels of 1.0–2.0 ng/mL and further increased to 95% for PSA > 2.0 ng/mL (42). However, the cut-off value for PSA performing PSMA PET has yet to be concluded, and thus prospective studies are required to recommend PSMA PET for patients with BCR.
Recently, a few studies raised possibilities that the detection rates of PSMA PET are also related with PSA doubling time. Aforementioned retrospective studies failed to prove the association between the detection rates and PSA doubling time in patients with BCR (2341). However, in a recent study analyzing 39 patients with a PSA level of less than 2 ng/mL, a strong relationship was found between the detection rates and PSA doubling time; the detection rates were 85% for a PSA doubling time of less than 6.5 months but only 19% for a PSA doubling time of greater than 6.5 months (43). In addition, higher sensitivities were noted in patients with shorter PSA doubling times and those with higher initial Gleason scores (44). Interpretation should be cautiously applied for both studies, since reliabilities are limited because of small number of study populations. Further studies with a larger number of patients are required to prove this relationship between the detection rates and PSA doubling time.
Potential roles of PSMA PET have yet to be fully investigated for other clinical applications, except patients with BCR after curative treatments. Recently, it has been suggested that PSMA PET is useful for primary staging in patients with high-risk PCa. Almost one third of PCa patients will experience a BCR within 10 years after primary treatment. Thus, it is of great concern for many clinicians to distinguish PCa with a malignant potential that will eventually recur despite successful local treatments, from other type of localized PCa. Accurate initial staging in consideration of malignant potential is one of the clinical unmet needs in the management of PCa, since it is a requisite for tailoring initial and subsequent treatment strategies. The diagnostic performances of 68Ga-PSMA PET for primary staging are yet to be proven, but several studies have shown promising results.
Primary staging is important to establish treatment strategies in PCa patients with high-risk disease before surgical procedures or external beam radiation therapy (Fig. 3). In particular, LN and bone metastases are likely to be present in patients with high-risk disease (Gleason score > 7, PSA > 20 ng/mL, clinical stage T2c-3a), even at the initial presentation. Although guidelines recommend to perform abdominal CT and bone scan for the evaluation of metastases in high-risk patients, diagnostic performances of these modalities are still poor (45). In patients with lower PSA levels, it is particularly difficult to detect metastases by conventional imaging modalities. The diagnostic accuracies for the detection of bone and LN metastases were associated with PSA levels in a meta-analysis evaluating 23 studies; 2.3% for PSA < 10 ng/mL, 5.3% for PSA 10.1–19.9 ng/mL, and 16.4% for PSA 20.0–49.9 ng/mL, respectively (12). The imaging modalities in the diagnosis of bone metastases were compared in a meta-analysis evaluating 27 studies, the pooled sensitivity and pooled specificity of bone scan with 99mTc-bisophophonate were 79% and 82%, respectively (46). The diagnostic accuracy of CT and MRI for the pelvic LN staging are so poor that the pooled sensitivity was 42% (95% confidential interval: 26–56%), and the pooled specificity was 82% (95% confidential interval: 80–83%), respectively, in a meta-analysis evaluating 24 studies (47).
Several studies have demonstrated the superiority of PSMA PET compared to conventional imaging modalities for the detection of LN metastases at primary staging. In a patient with high-risk disease, staging may be beneficial to provide a curative intent treatment plan, but the preoperative evaluation of LN metastasis by conventional imaging modalities has limited sensitivity and specificity. Conventional imaging modalities apply only size criteria to determine LN metastasis; pelvic LNs larger than 8 mm to 10 mm in diameter are usually considered suspicious (47). The sensitivity of CT and MRI in the LN detection was found to be < 40% using minimum size of 10 mm as a threshold, and about 80% of the pathologically-proven metastatic LNs were smaller than 8 mm (48). The sensitivity of morphological cross-sectional imaging was low without significant difference in the diagnostic performance between CT and MR (47). Therefore, pelvic LN dissection or sampling is considered to be the gold standard for the LN staging in PCa patients (4950).
In a meta-analysis analyzing 68Ga-PSMA PET, the pooled sensitivity for the LN detection was 61% and pooled specificity was 97% by patient-based analysis (42). Similar results were deducted by a prospective study performed in 30 patients with intermediate-to high-risk PCa using 68Ga-PSMA PET; sensitivity and specificity for the LN detection were 64% and 95%, respectively by per-patient analysis (51). In this study, the size dependence of positively imaged LN was suggested as the median size of metastatic deposit of true-positive LNs was significant larger than that of false-negative LNs (4.7 mm vs. 2.7 mm) in the histopathological evaluation. It is understandable considering of the spatial resolution of state-of-the-art PET cameras with 4.9 mm and 5.1 mm (52). Also, a close association was suggested between detection rates of the LN and the size of LNs. The median size of detected LNs was significantly larger than that of undetected LNs (13.6 mm vs. 4.3 mm) in a study using 68Ga-PSMA PET for the evaluation of patients with high-risk disease prior to RP (53). The detection of radiologically occult LN metastases can significantly influence patient management by modification of treatment plans, but the impact of improved sensitivity by 68Ga-PSMA PET on overall survival remains unanswered in patients with high-risk disease.
Prostate-specific membrane antigen PET is also expected to demonstrate a better diagnostic accuracy for the detection of bone metastases in primary staging. A comparison study was retrospectively performed in 126 patients, which revealed that a sensitivity and specificity of 98.8–99.0% and 98.9–100% for 68Ga-PSMA PET, and 82.4–86.6% and 91.6–97.9% for bone scan, respectively, by lesion-based analysis (20). In this study, 68Ga-PSMA PET showed better results in all subgroup analyses, except patient-based analysis in castration-resistant PCa (CRPCa). This PSMA finding suggested a loss of PSMA expression that is infrequently occurred in CRPCa patients (54). It seems relevant that bone scan or 18F-NaF PET are followed in order to assess PSMA-negative tumors or sclerotic bone lesions on PSMA PET.
However, the application of PSMA PET is limited for the evaluation of primary prostate tumor, because the spatial resolution of PET is relatively low and exact evaluation of the prostate area can be hampered by the retention of the PET tracer in the bladder. Combination with multi-parametric magnetic resonance imaging (mpMRI) or development of novel PSMA ligands with different biodistribution profile are suggested to enhance diagnostic accuracies of PSMA PET of primary tumor. In a retrospective study comparing 68Ga-PSMA PET and mpMRI, 68Ga-PSMA PET was well correlated with the tumor extension assessed in mpMRI in patients with a high pre-test probability for large primary tumor in the primary staging (55). Furthermore, simultaneous acquisition of 68Ga-PSMA PET with mpMRI was reported to improve the localization of primary tumor, and 68Ga-PSMA PET was more accurate in the primary tumor localization, as compared with mpMRI (56). Further details about the combination with PET and MRI will follow in the later section of this review.
Imaging guided targeted biopsy can be a possible application of PSMA PET at the initiation presentation. High false-negative results are inherent from the biopsy method and tumor heterogeneity of PCa, and targeted biopsy after negative result is often recommended to patients with high suspicion of PCa. Recently, a supplementary role of 68Ga-PSMA PET was suggested for the guidance of repeated biopsies in patients with high suspicion of PCa (56). In addition, 68Ga-PSMA PET-guided biopsy could be used for the surveillance of patients who already underwent multiple repeated biopsies. For this purpose, combination of 68Ga-PSMA PET with mpMRI was investigated to allow for the potential image-guided fusion biopsy incorporating information provided by mpMRI, which could lead to an increase of the diagnostic confidence (56).
Prostate-specific membrane antigen PET can assist salvage radiotherapy (SRT) by accurately delineating the target tumor volume. SRT is the main therapeutic option for patients with BCR after RP, which may potentially be curative or enhance the probability of progression-free survival (5758). It is essential to delineate clinical target volumes (CTVs) so as to include all the areas with potential microscopic occult tumors in SRT planning. The potential roles of PSMA PET have been investigated in several retrospective studies (5859), which is limited because of inhomogeneous study populations and study designs. Recently, a multicenter post hoc retrospective analysis of 68Ga-PSMA-11 PET was performed in 270 patients with early BCR at very low PSA levels < 1.0 ng/mL (60). It was reported that there was a major impact on SRT planning, which suggests the presence of at least one PSMA-positive lesion that was originally not covered by the consensus CTVs. However, it still remains unclear that expansion of radiation field could be beneficial in patients with BCR, putting up with additional toxicity. Further studies including randomized trials of SRT are required to evaluate potential benefits of PSMA PET in SRT planning.
Prostate-specific membrane antigen PET can be utilized in monitoring treatment response of PCa. Although Response Criteria in Solid Tumors (RECIST) guideline is commonly used for the response evaluation, revised RECIST 1.1 application is limited for the evaluation of PCa because of the high prevalence of non-measurable lesions in the LNs and bones. Currently, no reliable, objective surrogate marker is available for the response evaluation of metastatic PCa, considering that bone is the most frequently and almost exclusively metastasized sites. The diagnostic accuracies of bone scan or 18F-NaF PET for the response evaluation can be hampered by flare phenomenon. In this regard, PSMA PET can be a potential candidate for the monitoring of systemic disease. PET Response Criteria in Solid Tumors (PERCIST) was initially developed for systematic and structured assessment of response to therapy with 18F-fluorodeoxyglucose (61). In order to apply PSMA PET for the response evaluation of PCa, validation studies are required using PERCIST in PCa patients.
Another promising application of PSMA PET is the realization of theranosis. Therapeutics using PSMA ligands labeled with therapeutic radionuclides (177Lu or 90Y) has been developed for patients with advanced and metastatic PCa. Although PSMA-11 is the most widely investigated PSMA ligand for PET, but it is not available for RLT. PSMA-11 is unlikely to bind with other radiometals except 68Ga. Instead of PSMA-11, PSMA-617 labeled with a therapeutic radionuclide was developed for PSMA RLT (62). 177Lu-PSMA-617 is the most actively investigated radioligand for the treatment of PCa, and it has yielded promising outcomes in a multinational multicenter clinical trial in a large cohort of PCa patients (63). Accordingly, there is a growing experience regarding theranostic applications of PMSA PET. Multiple sessions of PSMA PET performed before and during PSMA RLT. In particular, PSMA PET is required for the patient selection before PSMA RLT, since it is crucial to determine the presence and intensity of PSMA expression in PCa patients (63646566). PSMA RLT can be futile, leaving immense complications without significant clinical benefits when given to patients presenting with a low level of PSMA expression.
There are several studies comparing 68Ga-PSMA PET to choline PET; however, data from prospective multicenter trials are not yet available. In a retrospective study evaluating 67 patients with 458 LN metastases, 68Ga-PSMA-11 demonstrated a higher detection rate in patients with BCR than that of 11C-choline (67); 39% were exclusively identified with 68Ga-PSMA-11, while 6% were identified with 11C-choline. There was a study comparing 18F-fluorocholine and 68Ga-PSMA PET, and these two PET scans performed within 30 days in 37 patients with BCR; 86.5% of tumors were detected by 68Ga-PSMA-11, and 70.3% were detected by 18F-fluorocholine by a patient-based analysis (26). In this study, 68Ga-PSMA-11 offered a higher detection rate, higher maximum standardized uptake value, and higher tumor-to-background ratio than 18F-fluorocholine. In another study with a prospective design demonstrated that 68Ga-PSMA exhibited a better diagnostic accuracy than did 18F-fluorocholine in patients with BCR (17).
There are several different PSMA ligands for 68Ga complexation; 68Ga-PSMA-11, 68Ga-PSMA-617, and 68Ga-PSMA-I&T. These PSMA ligands are collectively known as 68Ga-PSMA in this review, because they shared similar biodistribution and imaging properties. Studies discussed in the above section were mainly performed with 68Ga-PSMA-11 PET, but only a small number of studies have been performed using other 68Ga-PSMA ligands, and the difference in the diagnostic performances of 68Ga-PSMA ligands appears marginal. A retrospective study with 68Ga-PSMA-I&T reported detection rates of 52% for a PSA level of < 0.5 ng/mL, 55% for 0.5–1.0 ng/mL, 70% for 1.0–2.0 ng/mL, and 93% for 2.0–5.0 ng/mL; these results were relatively comparable to those for 68Ga-PSMA-11 (68). Notably, there are still no data directly comparing the 68Ga-PSMA ligands each other, and 68Ga-PSMA-11 is the most prominent PSMA ligand for PET imaging in PCa. The most suitable PSMA ligand for both diagnosis and treatment is yet to be determined, and investigations continue to accomplish an efficient and successful theranosis for PCa.
In general, 18F-based PET is known to be advantageous over 68Ga-based PET, but there are no head-to-head comparison studies comparing 68Ga-PSMA and 18F-PSMA PET. In a study evaluating 14 selected patients who underwent both 18F-DCFPyL PET and 68Ga-PSMA-11 PET, 18F-DCFPyL PET revealed additional lesions in 3 out of 14 patients in whom 68Ga-PSMA-11 PET revealed negative and inconclusive findings (69). In a follow-up study performed by the same group, PSA-stratified detection rates were compared in different patients and the diagnostic accuracy of 18F-DCFPyL PET was non-inferior to that of 68Ga-PSMA-11 PET (70). These initial data suggested that 18F-PSMA PET might have improved sensitivity for the detection of relapsed tumor in patients with BCR after RP with moderately increased PSA levels. However, these data should be interpreted with caution because of the different patient populations, administered activities, and PET acquisition techniques. Further studies are required to confirm the potential superiority of 18F-PSMA PET over other imaging modalities.
MRI is considered to be the standard imaging tool for the evaluation of soft tissues, but MRI remains challenging for the diagnosis of PCa. mpMRI is accurate and useful to evaluate PCa, since it combines conventional anatomical imaging based on T2-weighted sequences and functional imaging such as diffuse-weighted sequences. mpMRI has been integrated into the management of PCa, in particular for assisting prostate biopsies through image guidance. It has been demonstrated that mpMRI-guided prostate biopsies enhanced diagnostic yields for patients with clinically significant PCa, which lead to improvement of patient care (7172). However, the reported diagnostic performances of T2-weighted sequences varied widely in the detection of PCa, with sensitivities of 47.8–88.2% and specificities of 44.3–81.0%, respectively (73). Treatment-induced changes such as distorted anatomy, fibrosis, artifacts can contribute misinterpretations of MRI findings, and thus hinder the diagnostic accuracy. In addition, the widespread use of mpMRI has been hampered by its relatively high cost, its complexity, and lack of training.
Recently, technological progress including simultaneous acquisition of PET and MRI (hybrid PET/MRI) has lead the active use of PET/MRI. Functional information provided by PET is expected to enhance diagnostic performances of conventional anatomical MRI. The hybrid PET/MRI allows optimal conditions for the registration of the two modalities, and thus both PET and MRI data can be reliably acquired in a single session. Moreover, the hybrid PET/MRI enables the whole-body survey in one examination without additional radiation originating from the use of CT. In this respect, the hybrid PET/MRI provides a potential added value of combined MRI and PET over mpMRI alone or PET/CT.
The feasibility of the hybrid PET/MRI has been investigated with various prostate specific PET tracers. The diagnostic benefits of the hybrid PET/MRI with the clinically established choline tracers were suggested in the detection of PCa (7475). The diagnostic performances of the hybrid PET/MR with 18F-fluorocholine and 11C-choline was compared with that of mpMRI alone, which showed a better diagnostic accuracy in the peripheral zone than in the transition zone of the prostate (75). PSMA-targeting PET tracers have also been explored for the implementation of the hybrid PET/MRI. In the diagnosis of recurrent PCa, 68Ga-PSMA PET/MRI detected PCa more easily and more accurately than 68Ga-PSMA PET/CT, and unclear findings on PET/CT could be clarified by PET/MRI (76). Also, 68Ga-PSMA PET/MRI improved the localization of primary PCa, compared both with mpMRI and with PET alone (56). Recently, 18F-PSMA-1007 was applied for the hybrid PET/MRI system in PCa patients (77). In this study, 18F-PSMA-1007 presented better imaging features compared with those of 68Ga-PSMA, which is mainly due to improved pharmacokinetics of 18F-PSMA-1007 and optimized imaging protocols for the hybrid PET/MRI.
The hybrid PET/MRI with prostate specific PET tracers is a promising tool to add potential values in the diagnosis of PCa, and it is also expected to significantly influence on the management of PCa patients. However, further technological advances including optimization of imaging protocols and selection of the best suited PET tracer are needed to become a preferred imaging tool for PCa. In addition, prospective studies using the hybrid PET/MRI with prostate specific PET tracers are required, particularly in comparison with other imaging modalities such as PET/CT and mpMRI alone.
There are several limitations in PSMA PET stemming from characteristics of PSMA ligands and PCa biology. 68Ga-PSMA PET can produce false-negatives in up to 10% of patients with primary PCa (7879). However, the underlying etiology of PCa with negative findings on PSMA PET is still unclear, given the lack of prospective studies and correlation with immunohistochemistry. In addition, it has been reported that in advanced metastatic CRPCa, metastases (mainly in the liver) can lose PSMA expression (54). The evaluation of the prostate area can be disturbed by the activity 68Ga-PSMA-11 in the urinary bladder. A whole-body scan usually starts at 60 minutes post-tracer injection (p.i.) of 68Ga-PSMA-11 in the clinical routine, and the identification of local recurrence may be hampered due to high physiologic urinary bladder activity at this time point. It is known that tumor uptake of 68Ga-PSMA-11 occurs earlier than tracer accumulation in the urinary bladder. In this regard, additional dynamic imaging acquisition during the first 8 minutes p.i. was suggested as an alternative, and the detection rate of local recurrence increased in patients with BCR (80).
Despite enthusiasms and scientific efforts to drive PSMA researches that enables huge number of publications in a short period time, many challenges are awaiting PSMA PET to be incorporated into the clinical practice. However, evidence is still limited for the clinical applications of PSMA PET. Improved diagnosis by PSMA PET is expected to lead improvements in the management of patients with high-risk disease, but the impact of the increased metastatic LN detection on the overall survival remains unanswered. At this early stage, prospective multicenter trials are needed to validate the effectiveness and usefulness of PSMA PET.
Prostate-specific membrane antigen PET has emerged as a promising, accurate method for the detection of tumor recurrence in patients with BCR. Patients in this clinical situation could get considerable benefits from PSMA PET to localize the origin of the PSA increase, even at very low PSA levels. PSMA PET also has important clinical utilities in primary staging, treatment planning, response evaluation, and PSMA RLT. Nevertheless, prospective multicenter trials are needed to confirm the effectiveness and usefulness of PSMA PET.
Figures and Tables
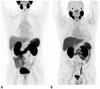 | Fig. 1Representative images of PSMA PET.
A. 68Ga-PSMA-11 PET shows normal biodistribution of PSMA across body; lacrimal and salivary glands, liver, spleen, kidneys, and intestines. B. 18F-DCFPyL PET demonstrates normal biodistribution of PSMA which is similar to 68Ga-PSMA-11 PET with better image resolution. These images were reprinted with permissions from reference articles 28 and 81, respectively. Adapted from Fendler et al. Eur J Nucle Med Mol Imaging 2017;44:1014-1024, 2017;44:2117-2136 [28] and Sheikhbahaei et al. Eur J Nucle Med Mol Imaging 2017;44:2117-2136 [81], with permissions of Springer Science and Bus Media B V. PSMA = prostate-specific membrane antigen
|
 | Fig. 2PSA level and 68Ga-PSMA PET positivity.Scatterplot shows association between PSA level and 68Ga-PSMA PET positivity. Red line is meta-regression prediction and shading shows 95% confidence interval. Size of circles is related to inverse of variance. This image was reprinted with permission from reference article 42. Adapted from Perera et al. Eur Urol 2016;70:926-937, with permission of Elsevier Science and Technology Journals [42]. PSA = prostate specific antigen
|
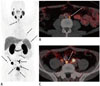 | Fig. 3Potential benefits of PSMA PET for treatment planning.
68Ga-PSMA-I&T PET/CT was performed in prostate cancer patient (Gleason Scores 4 + 5 = 9) who planned external beam radiation therapy after radical prostatectomy 1 month ago, and PSA level was 0.37 ng/mL at time of PET scanning. Maximum intensity projection shows multiple uptakes (arrows) suggestive of metastases (A), and axial fusion PET/CT images demonstrate metastatic retroperitoneal lymph nodes (arrows) with diameters of 3 mm (B) and 9 mm (C). These images were provided by courtesy of Professor Dr. Richard P. Baum at THERANOSTICS Center for Molecular Radiotherapy & Molecular Imaging, Zentralklinik Bad Berka, Germany.
|
References
1. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017; 3:524–548.
2. Dong JT, Rinker-Schaeffer CW, Ichikawa T, Barrett JC, Isaacs JT. Prostate cancer--biology of metastasis and its clinical implications. World J Urol. 1996; 14:182–189.

3. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998; 280:969–974.
4. Serefoglu EC, Altinova S, Ugras NS, Akincioglu E, Asil E, Balbay MD. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can Urol Assoc J. 2013; 7:E293–E298.

5. Bjurlin MA, Carter HB, Schellhammer P, Cookson MS, Gomella LG, Troyer D, et al. Optimization of initial prostate biopsy in clinical practice: sampling, labeling and specimen processing. J Urol. 2013; 189:2039–2046.

6. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997; 3:81–85.
7. Bostwick DG, Pacelli A, Blute M, Roche P, Murphy GP. Prostate specific membrane antigen expression in prostatic intraepithelial neoplasia and adenocarcinoma: a study of 184 cases. Cancer. 1998; 82:2256–2261.
8. Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004; 91:528–539.

9. Evans MJ, Smith-Jones PM, Wongvipat J, Navarro V, Kim S, Bander NH, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci U S A. 2011; 108:9578–9582.

10. Ross JS, Sheehan CE, Fisher HA, Kaufman RP Jr, Kaur P, Gray K, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003; 9:6357–6362.
11. Troyer JK, Beckett ML, Wright GL Jr. Detection and characterization of the prostate-specific membrane antigen (PSMA) in tissue extracts and body fluids. Int J Cancer. 1995; 62:552–558.
12. Abuzallouf S, Dayes I, Lukka H. Baseline staging of newly diagnosed prostate cancer: a summary of the literature. J Urol. 2004; 171(6 Pt 1):2122–2127.

13. Ackerstaff E, Pflug BR, Nelson JB, Bhujwalla ZM. Detection of increased choline compounds with proton nuclear magnetic resonance spectroscopy subsequent to malignant transformation of human prostatic epithelial cells. Cancer Res. 2001; 61:3599–3603.
14. Reske SN, Blumstein NM, Neumaier B, Gottfried HW, Finsterbusch F, Kocot D, et al. Imaging prostate cancer with 11C-choline PET/CT. J Nucl Med. 2006; 47:1249–1254.
15. Farsad M, Schiavina R, Castellucci P, Nanni C, Corti B, Martorana G, et al. Detection and localization of prostate cancer: correlation of (11)C-choline PET/CT with histopathologic step-section analysis. J Nucl Med. 2005; 46:1642–1649.
16. Sutinen E, Nurmi M, Roivainen A, Varpula M, Tolvanen T, Lehikoinen P, et al. Kinetics of [(11)C]choline uptake in prostate cancer: a PET study. Eur J Nucl Med Mol Imaging. 2004; 31:317–324.
17. Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015; 56:1185–1190.
18. Herlemann A, Wenter V, Kretschmer A, Thierfelder KM, Bartenstein P, Faber C, et al. 68Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016; 70:553–557.
19. Hijazi S, Meller B, Leitsmann C, Strauss A, Meller J, Ritter CO, et al. Pelvic lymph node dissection for nodal oligometastatic prostate cancer detected by 68Ga-PSMA-positron emission tomography/computerized tomography. Prostate. 2015; 75:1934–1940.
20. Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016; 43:2114–2121.
21. Ren J, Yuan L, Wen G, Yang J. The value of anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT in the diagnosis of recurrent prostate carcinoma: a meta-analysis. Acta Radiol. 2016; 57:487–493.

22. Pandit-Taskar N, O'Donoghue JA, Durack JC, Lyashchenko SK, Cheal SM, Beylergil V, et al. A phase I/II study for analytic validation of 89Zr-J591 immunoPET as a molecular imaging agent for metastatic prostate cancer. Clin Cancer Res. 2015; 21:5277–5285.
23. Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015; 42:197–209.

24. Chen Y, Foss CA, Byun Y, Nimmagadda S, Pullambhatla M, Fox JJ, et al. Radiohalogenated prostate-specific membrane antigen (PSMA)-based ureas as imaging agents for prostate cancer. J Med Chem. 2008; 51:7933–7943.

25. Eder M, Schäfer M, Bauder-Wüst U, Hull WE, Wängler C, Mier W, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012; 23:688–697.
26. Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68) Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014; 41:11–20.
27. Afshar-Oromieh A, Hetzheim H, Kübler W, Kratochwil C, Giesel FL, Hope TA, et al. Radiation dosimetry of (68)Ga-PSMA-11 (HBED-CC) and preliminary evaluation of optimal imaging timing. Eur J Nucl Med Mol Imaging. 2016; 43:1611–1620.

28. Fendler WP, Eiber M, Beheshti M, Bomanji J, Ceci F, Cho S, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017; 44:1014–1024.
29. Prasad V, Steffen IG, Diederichs G, Makowski MR, Wust P, Brenner W. Biodistribution of [(68)Ga]PSMA-HBED-CC in patients with prostate cancer: characterization of uptake in normal organs and tumour lesions. Mol Imaging Biol. 2016; 18:428–436.

30. Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013; 40:486–495.
31. Cho SY, Gage KL, Mease RC, Senthamizhchelvan S, Holt DP, Jeffrey-Kwanisai A, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012; 53:1883–1891.
32. Chen Y, Pullambhatla M, Foss CA, Byun Y, Nimmagadda S, Senthamizhchelvan S, et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011; 17:7645–7653.
33. Szabo Z, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015; 17:565–574.
34. Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017; 44:678–688.

35. Suardi N, Gandaglia G, Gallina A, Di Trapani E, Scattoni V, Vizziello D, et al. Long-term outcomes of salvage lymph node dissection for clinically recurrent prostate cancer: results of a single-institution series with a minimum follow-up of 5 years. Eur Urol. 2015; 67:299–309.

36. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. European Association of Urology. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014; 65:124–137.

37. Sella T, Schwartz LH, Swindle PW, Onyebuchi CN, Scardino PT, Scher HI, et al. Suspected local recurrence after radical prostatectomy: endorectal coil MR imaging. Radiology. 2004; 231:379–385.

38. Casciani E, Polettini E, Carmenini E, Floriani I, Masselli G, Bertini L, et al. Endorectal and dynamic contrast-enhanced MRI for detection of local recurrence after radical prostatectomy. AJR Am J Roentgenol. 2008; 190:1187–1192.

39. Cirillo S, Petracchini M, Scotti L, Gallo T, Macera A, Bona MC, et al. Endorectal magnetic resonance imaging at 1.5 Tesla to assess local recurrence following radical prostatectomy using T2-weighted and contrast-enhanced imaging. Eur Radiol. 2009; 19:761–769.

40. Panebianco V, Sciarra A, Lisi D, Galati F, Buonocore V, Catalano C, et al. Prostate cancer: 1HMRS-DCEMR at 3T versus [(18)F]choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after radical retropubic prostatectomy (RRP). Eur J Radiol. 2012; 81:700–708.

41. Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015; 56:668–674.
42. Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016; 70:926–937.
43. Ceci F, Uprimny C, Nilica B, Geraldo L, Kendler D, Kroiss A, et al. (68)Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015; 42:1284–1294.

44. Verburg FA, Pfister D, Heidenreich A, Vogg A, Drude NI, Vöö S, et al. Extent of disease in recurrent prostate cancer determined by [(68)Ga]PSMA-HBED-CC PET/CT in relation to PSA levels, PSA doubling time and Gleason score. Eur J Nucl Med Mol Imaging. 2016; 43:397–403.

45. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017; 71:618–629.
46. Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol. 2014; 43:1503–1513.

47. Hövels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008; 63:387–395.

48. Heesakkers RA, Hövels AM, Jager GJ, van den Bosch HC, Witjes JA, Raat HP, et al. MRI with a lymph-node-specific contrast agent as an alternative to CT scan and lymph-node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol. 2008; 9:850–856.

49. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, et al. European Association of Urology. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014; 65:467–479.

50. Briganti A, Blute ML, Eastham JH, Graefen M, Heidenreich A, Karnes JR, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009; 55:1251–1265.

51. van Leeuwen PJ, Emmett L, Ho B, Delprado W, Ting F, Nguyen Q, et al. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017; 119:209–215.
52. Kolthammer JA, Su KH, Grover A, Narayanan M, Jordan DW, Muzic RF. Performance evaluation of the Ingenuity TF PET/CT scanner with a focus on high count-rate conditions. Phys Med Biol. 2014; 59:3843–3859.

53. Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016; 69:393–396.

54. Laidler P, Dulińska J, Lekka M, Lekki J. Expression of prostate specific membrane antigen in androgen-independent prostate cancer cell line PC-3. Arch Biochem Biophys. 2005; 435:1–14.

55. Giesel FL, Sterzing F, Schlemmer HP, Holland-Letz T, Mier W, Rius M, et al. Intra-individual comparison of (68)Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur J Nucl Med Mol Imaging. 2016; 43:1400–1406.

56. Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol. 2016; 70:829–836.
57. Zumsteg ZS, Daskivich TJ, Sandler HM. Salvage radiotherapy for biochemically recurrent prostate cancer after prostatectomy. J Clin Oncol. 2016; 34:3829–3833.

58. Goenka A, Magsanoc JM, Pei X, Schechter M, Kollmeier M, Cox B, et al. Long-term outcomes after high-dose postprostatectomy salvage radiation treatment. Int J Radiat Oncol Biol Phys. 2012; 84:112–118.

59. Valicenti RK, Thompson I Jr, Albertsen P, Davis BJ, Goldenberg SL, Wolf JS, et al. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int J Radiat Oncol Biol Phys. 2013; 86:822–828.

60. HCalais J, Czernin J, Cao M, Kishan AU, Hegde JV, Shaverdian N, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence following radical prostatectomy in 270 patients with PSA < 1.0 ng/ml: impact on salvage radiotherapy planning. J Nucl Med. 2018; 59:230–237.
61. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009; 50:Suppl 1. 122S–150S.

62. Afshar-Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med. 2015; 56:1697–1705.

63. Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017; 58:85–90.
64. Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med. 2016; 57:1334–1338.
65. Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016; 57:1006–1013.
66. Ahmadzadehfar H, Eppard E, Kürpig S, Fimmers R, Yordanova A, Schlenkhoff CD, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016; 7:12477–12488.
67. Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017; 44:92–101.
68. Berliner C, Tienken M, Frenzel T, Kobayashi Y, Helberg A, Kirchner U, et al. Detection rate of PET/CT in patients with biochemical relapse of prostate cancer using [68Ga]PSMA I&T and comparison with published data of [68Ga]PSMA HBED-CC. Eur J Nucl Med Mol Imaging. 2017; 44:670–677.
69. Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, Schomäcker K, et al. Comparison of [(18)F]DCFPyL and [ (68) Ga]Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015; 17:575–584.
70. Dietlein F, Kobe C, Neubauer S, Schmidt M, Stockter S, Fischer T, et al. PSA-stratified performance of 18F- and 68Ga-PSMA PET in patients with biochemical recurrence of prostate cancer. J Nucl Med. 2017; 58:947–952.
71. Hoeks CM, Schouten MG, Bomers JG, Hoogendoorn SP, Hulsbergen-van de Kaa CA, Hambrock T, et al. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol. 2012; 62:902–909.

72. Puech P, Rouvière O, Renard-Penna R, Villers A, Devos P, Colombel M, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy--prospective multicenter study. Radiology. 2013; 268:461–469.

73. Yakar D, Debats OA, Bomers JG, Schouten MG, Vos PC, van Lin E, et al. Predictive value of MRI in the localization, staging, volume estimation, assessment of aggressiveness, and guidance of radiotherapy and biopsies in prostate cancer. J Magn Reson Imaging. 2012; 35:20–31.

74. Souvatzoglou M, Eiber M, Takei T, Fürst S, Maurer T, Gaertner F, et al. Comparison of integrated whole-body [11C]choline PET/MR with PET/CT in patients with prostate cancer. Eur J Nucl Med Mol Imaging. 2013; 40:1486–1499.

75. de Perrot T, Rager O, Scheffler M, Lord M, Pusztaszeri M, Iselin C, et al. Potential of hybrid 18F-fluorocholine PET/MRI for prostate cancer imaging. Eur J Nucl Med Mol Imaging. 2014; 41:1744–1755.
76. Afshar-Oromieh A, Haberkorn U, Schlemmer HP, Fenchel M, Eder M, Eisenhut M, et al. Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging. 2014; 41:887–897.
77. Freitag MT, Kesch C, Cardinale J, Flechsig P, Floca R, Eiber M, et al. Simultaneous whole-body 18F-PSMA-1007-PET/MRI with integrated high-resolution multiparametric imaging of the prostatic fossa for comprehensive oncological staging of patients with prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2018; 45:340–347.

78. Budäus L, Leyh-Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, et al. Initial experience of (68)Ga-PSMA PET/CT imaging in high-risk prostate cancer patients prior to radical prostatectomy. Eur Urol. 2016; 69:393–396.

79. Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016; 195:1436–1443.
80. Uprimny C, Kroiss AS, Decristoforo C, Fritz J, Warwitz B, Scarpa L, et al. Early dynamic imaging in 68Ga-PSMA-11 PET/CT allows discrimination of urinary bladder activity and prostate cancer lesions. Eur J Nucl Med Mol Imaging. 2017; 44:765–775.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download