Introduction
Positioning of LABA/LAMA in COPD Treatment Guidelines
Overview of Key Trials of LABA/LAMA FDCs
Table 1
Approved LABA/LAMA FDCs

*Not approved in Korea.
LABA/LAMA: long-acting β2-agonist/long-acting muscarinic antagonist; FDC: fixed-dose combination; IND/GLY: indacaterol/glycopyrronium; q.d.: once daily; VI/UMEC: vilanterol/umeclidinium; COPD: chronic obstructive pulmonary disease; OLO/TIO: olodaterol/tiotropium; FOR/ACLI: formoterol/aclidinium; b.i.d.: twice daily; FF/GP: formoterol fumarate/glycopyrrolate.
Table 2
Overview of completed Phase III studies of LABA/LAMA FDCs by the primary endpoint
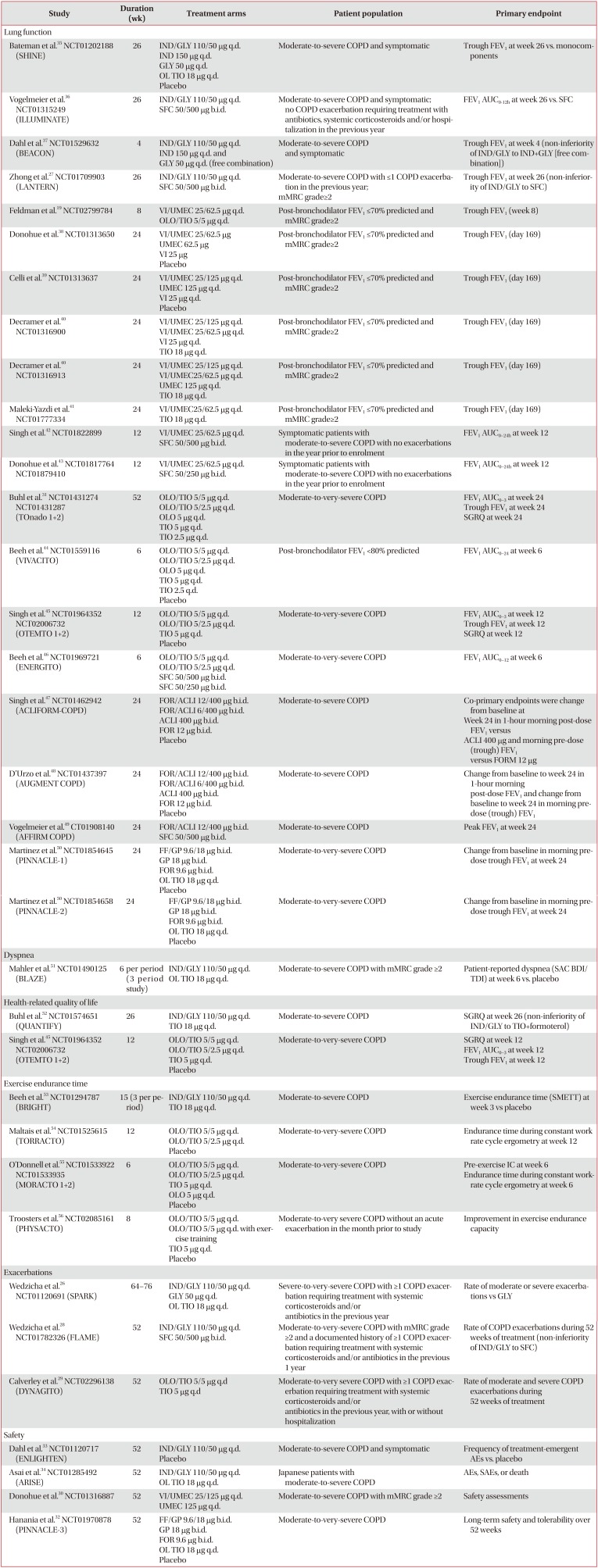
| Study | Duration (wk) | Treatment arms | Patient population | Primary endpoint |
|---|---|---|---|---|
| Lung function | ||||
| Bateman et al.35 NCT01202188 (SHINE) | 26 | IND/GLY 110/50 µg q.d. | Moderate-to-severe COPD and symptomatic | Trough FEV1 at week 26 vs. monocomponents |
| IND 150 µg q.d. | ||||
| GLY 50 µg q.d. | ||||
| OL TIO 18 µg q.d. | ||||
| Placebo | ||||
| Vogelmeier et al.36 NCT01315249 (ILLUMINATE) | 26 | IND/GLY 110/50 µg q.d. | Moderate-to-severe COPD and symptomatic; no COPD exacerbation requiring treatment with antibiotics, systemic corticosteroids and/or hospitalization in the previous year | FEV1 AUC0–12h at week 26 vs. SFC |
| SFC 50/500 µg b.i.d. | ||||
| Dahl et al.37 NCT01529632 (BEACON) | 4 | IND/GLY 110/50 µg q.d. | Moderate-to-severe COPD and symptomatic | Trough FEV1 at week 4 (non-inferiority of IND/GLY to IND+GLY [free combination]) |
| IND 150 µg q.d. and | ||||
| GLY 50 µg q.d. (free combination) | ||||
| Zhong et al.27 NCT01709903 (LANTERN) | 26 | IND/GLY 110/50 µg q.d. | Moderate-to-severe COPD with ≤1 COPD exacerbation in the previous year; mMRC grade≥2 | Trough FEV1 at week 26 (non-inferiority of IND/GLY to SFC) |
| SFC 50/500 µg b.i.d. | ||||
| Feldman et al.19 NCT02799784 | 8 | VI/UMEC 25/62.5 µg q.d. | Post-bronchodilator FEV1 ≤70% predicted and mMRC grade≥2 | Trough FEV1 (week 8) |
| OLO/TIO 5/5 µg q.d. | ||||
| Donohue et al.38 NCT01313650 | 24 | VI/UMEC 25/62.5 µg | Post-bronchodilator FEV1 ≤70% predicted and mMRC grade≥2 | Trough FEV1 (day 169) |
| UMEC 62.5 µg | ||||
| VI 25 µg | ||||
| Placebo | ||||
| Celli et al.39 NCT01313637 | 24 | VI/UMEC 25/125 µg q.d. | Post-bronchodilator FEV1 ≤70% predicted and mMRC grade≥2 | Trough FEV1 (day 169) |
| UMEC 125 µg q.d. | ||||
| VI 25 µg q.d. | ||||
| Placebo | ||||
| Decramer et al.40 NCT01316900 | 24 | VI/UMEC 25/125 µg q.d. | Post-bronchodilator FEV1 ≤70% predicted and mMRC grade≥2 | Trough FEV1 (day 169) |
| VI/UMEC 25/62.5 µg q.d. | ||||
| VI 25 µg q.d. | ||||
| TIO 18 µg q.d. | ||||
| Decramer et al.40 NCT01316913 | 24 | VI/UMEC 25/125 µg q.d. | Post-bronchodilator FEV1 ≤70% predicted and mMRC grade≥2 | Trough FEV1 (day 169) |
| VI/UMEC25/62.5 µg q.d. | ||||
| UMEC 125 µg q.d. | ||||
| TIO 18 µg q.d. | ||||
| Maleki-Yazdi et al.41 NCT01777334 | 24 | VI/UMEC25/62.5 µg q.d. | Post-bronchodilator FEV1 ≤70% predicted and mMRC grade≥2 | Trough FEV1 (day 169) |
| TIO 18 µg q.d. | ||||
| Singh et al.42 NCT01822899 | 12 | VI/UMEC 25/62.5 µg q.d. | Symptomatic patients with moderate-to-severe COPD with no exacerbations in the year prior to enrolment | FEV1 AUC0–24h at week 12 |
| SFC 50/500 µg b.i.d. | ||||
| Donohue et al.43 NCT01817764 NCT01879410 | 12 | VI/UMEC 25/62.5 µg q.d. | Symptomatic patients with moderate-to-severe COPD with no exacerbations in the year prior to enrolment | FEV1 AUC0–24h at week 12 |
| SFC 50/250 µg b.i.d. | ||||
| Buhl et al.31 NCT01431274 NCT01431287 (TOnado 1+2) | 52 | OLO/TIO 5/5 µg q.d. | Moderate-to-very-severe COPD | FEV1 AUC0–3 at week 24 |
| OLO/TIO 5/2.5 µg q.d. | Trough FEV1 at week 24 | |||
| OLO 5 µg q.d. | SGRQ at week 24 | |||
| TIO 5 µg q.d. | ||||
| TIO 2.5 µg q.d. | ||||
| Beeh et al.44 NCT01559116 (VIVACITO) | 6 | OLO/TIO 5/5 µg q.d. | Post-bronchodilator FEV1 <80% predicted | FEV1 AUC0–24 at week 6 |
| OLO/TIO 5/2.5 µg q.d. | ||||
| OLO 5 µg q.d. | ||||
| TIO 5 µg q.d. | ||||
| TIO 2.5 q.d. | ||||
| Placebo | ||||
| Singh et al.45 NCT01964352 NCT02006732 (OTEMTO 1+2) | 12 | OLO/TIO 5/5 µg q.d. | Moderate-to-very-severe COPD | FEV1 AUC0–3 at week 12 |
| OLO/TIO 5/2.5 µg q.d. | Trough FEV1 at week 12 | |||
| TIO 5 µg q.d. | SGRQ at week 12 | |||
| Placebo | ||||
| Beeh et al.46 NCT01969721 (ENERGITO) | 6 | OLO/TIO 5/5 µg q.d. | Moderate-to-very-severe COPD | FEV1 AUC0–12 at week 6 |
| OLO/TIO 5/2.5 µg q.d. | ||||
| SFC 50/500 µg b.i.d. | ||||
| SFC 50/250 µg b.i.d. | ||||
| Singh et al.47 NCT01462942 (ACLIFORM-COPD) | 24 | FOR/ACLI 12/400 µg b.i.d. | Moderate-to-severe COPD | Co-primary endpoints were change from baseline at |
| FOR/ACLI 6/400 µg b.i.d. | Week 24 in 1-hour morning post-dose FEV1 versus | |||
| ACLI 400 µg b.i.d. | ACLI 400 µg and morning pre-dose (trough) FEV1 versus FORM 12 µg | |||
| FOR 12 µg b.i.d. | ||||
| Placebo | ||||
| D'Urzo et al.48 NCT01437397 (AUGMENT COPD) | 24 | FOR/ACLI 12/400 µg b.i.d. | Moderate-to-severe COPD | Change from baseline to week 24 in 1-hour morning post-dose FEV1 and change from baseline to week 24 in morning predose (trough) FEV1 |
| FOR/ACLI 6/400 µg b.i.d. | ||||
| ACLI 400 µg b.i.d. | ||||
| FOR 12 µg b.i.d. | ||||
| Placebo | ||||
| Vogelmeier et al.49 CT01908140 (AFFIRM COPD) | 24 | FOR/ACLI 12/400 µg b.i.d. | Moderate-to-severe COPD | Peak FEV1 at week 24 |
| SFC 50/500 µg b.i.d. | ||||
| Martinez et al.50 NCT01854645 (PINNACLE-1) | 24 | FF/GP 9.6/18 µg b.i.d. | Moderate-to-very-severe COPD | Change from baseline in morning predose trough FEV1 at week 24 |
| GP 18 µg b.i.d. | ||||
| FOR 9.6 µg b.i.d. | ||||
| OL TIO 18 µg q.d. | ||||
| Placebo | ||||
| Martinez et al.50 NCT01854658 (PINNACLE-2) | 24 | FF/GP 9.6/18 µg b.i.d. | Moderate-to-very-severe COPD | Change from baseline in morning predose trough FEV1 at week 24 |
| GP 18 µg b.i.d. | ||||
| FOR 9.6 µg b.i.d. | ||||
| OL TIO 18 µg q.d. | ||||
| Placebo | ||||
| Dyspnea | ||||
| Mahler et al.51 NCT01490125 (BLAZE) | 6 per period (3 period study) | IND/GLY 110/50 µg q.d. | Moderate-to-severe COPD with mMRC grade ≥2 | Patient-reported dyspnea (SAC BDI/TDI) at week 6 vs. placebo |
| OL TIO 18 µg q.d. | ||||
| Health-related quality of life | ||||
| Buhl et al.52 NCT01574651 (QUANTIFY) | 26 | IND/GLY 110/50 µg q.d. | Moderate-to-severe COPD | SGRQ at week 26 (non-inferiority of IND/GLY to TIO+formoterol) |
| TIO 18 µg q.d. | ||||
| Singh et al.45 NCT01964352 NCT02006732 (OTEMTO 1+2) | 12 | OLO/TIO 5/5 µg q.d. | Moderate-to-very-severe COPD | SGRQ at week 12 |
| OLO/TIO 5/2.5 µg q.d. | FEV1 AUC0–3 at week 12 | |||
| TIO 5 µg q.d. | Trough FEV1 at week 12 | |||
| Placebo | ||||
| Exercise endurance time | ||||
| Beeh et al.53 NCT01294787 (BRIGHT) | 15 (3 per period) | IND/GLY 110/50 µg q.d. | Moderate-to-severe COPD | Exercise endurance time (SMETT) at week 3 vs placebo |
| TIO 18 µg q.d. | ||||
| Maltais et al.54 NCT01525615 (TORRACTO) | 12 | OLO/TIO 5/5 µg q.d. | Moderate-to-very-severe COPD | Endurance time during constant work rate cycle ergometry at week 12 |
| OLO/TIO 5/2.5 µg q.d. | ||||
| Placebo | ||||
| O'Donnell et al.55 NCT01533922 NCT01533935 (MORACTO 1+2) | 6 | OLO/TIO 5/5 µg q.d. | Moderate-to-very-severe COPD | Pre-exercise IC at week 6 |
| OLO/TIO 5/2.5 µg q.d. | Endurance time during constant workrate cycle ergometry at week 6 | |||
| TIO 5 µg q.d. | ||||
| OLO 5 µg q.d. | ||||
| Placebo | ||||
| Troosters et al.56 NCT02085161 (PHYSACTO) | 8 | OLO/TIO 5/5 µg q.d. | Moderate-to-very severe COPD without an acute exacerbation in the month prior to study | Improvement in exercise endurance capacity |
| OLO/TIO 5/5 µg q.d. with exercise training | ||||
| TIO 5 µg q.d. | ||||
| Placebo | ||||
| Exacerbations | ||||
| Wedzicha et al.26 NCT01120691 (SPARK) | 64–76 | IND/GLY 110/50 µg q.d. | Severe-to-very-severe COPD with ≥1 COPD exacerbation requiring treatment with systemic corticosteroids and/or antibiotics in the previous year | Rate of moderate or severe exacerbations vs GLY |
| GLY 50 µg q.d. | ||||
| OL TIO 18 µg q.d. | ||||
| Wedzicha et al.28 NCT01782326 (FLAME) | 52 | IND/GLY 110/50 µg q.d. | Moderate-to-very-severe COPD with mMRC grade ≥2 and a documented history of ≥1 COPD exacerbation requiring treatment with systemic corticosteroids and/or antibiotics in the previous 1 year | Rate of COPD exacerbations during 52 weeks of treatment (non-inferiority of IND/GLY to SFC) |
| SFC 50/500 µg b.i.d. | ||||
| Calverley et al.29 NCT02296138 (DYNAGITO) | 52 | OLO/TIO 5/5 µg q.d | Moderate-to-very severe COPD with ≥1 COPD exacerbation requiring treatment with systemic corticosteroids and/or antibiotics in the previous year, with or without hospitalization | Rate of moderate and severe COPD exacerbations during 52 weeks of treatment |
| TIO 5 µg q.d | ||||
| Safety | ||||
| Dahl et al.33 NCT01120717 (ENLIGHTEN) | 52 | IND/GLY 110/50 µg q.d. | Moderate-to-severe COPD and symptomatic | Frequency of treatment-emergent AEs vs. placebo |
| Placebo | ||||
| Asai et al.34 NCT01285492 (ARISE) | 52 | IND/GLY 110/50 µg q.d. | Japanese patients with moderate-to-severe COPD | AEs, SAEs, or death |
| OL TIO 18 µg q.d. | ||||
| Donohue et al.30 NCT01316887 | 52 | VI/UMEC 25/125 µg q.d. | Moderate-to-severe COPD with mMRC grade ≥2 | Safety assessments |
| UMEC 125 µg q.d. | ||||
| Hanania et al.32 NCT01970878 (PINNACLE-3) | 52 | FF/GP 9.6/18 µg b.i.d. | Moderate-to-very-severe COPD | Long-term safety and tolerability over 52 weeks |
| GP 18 µg b.i.d. | ||||
| FOR 9.6 µg b.i.d. | ||||
| OL TIO 18 µg q.d. | ||||
| Placebo |
LABA/LAMA: long-acting β2-agonist/long-acting muscarinic antagonist; FDC: fixed-dose combination; IND/GLY: indacaterol/glycopyrronium; q.d.: once daily; IND: indacaterol; GLY: glycopyrronium; OL: open label; TIO: tiotropium; COPD: chronic obstructive pulmonary disease; FEV1: forced expiratory volume in 1 second; SFC: salmeterol/fluticasone; b.i.d.: twice daily; AUC: area under the curve; mMRC: modified Medical Research Council; VI/UMEC: vilanterol/umeclidinium; OLO/TIO: olodaterol/tiotropium; UMEC: umeclidinium; VI: vilanterol; FOR/ACLI: formoterol/aclidinium; ACLI: aclidinium; FOR: formoterol; FF/GP: formoterol fumarate/glycopyrrolate; GP: glycopyrrolate; SGRQ: St. George's Respiratory Questionnaire; BDI: baseline dyspnea index; TDI: transition dyspnea index;AEs: adverse events; SAEs: serious adverse events.
Clinical Trial Evidence: Efficacy of LABA/LAMA
1. Lung function
Table 3
Improvement in lung function with LABA/LAMA FDCs versus placebo, TIO and SFC2627283132333536384041424344454647484950515355
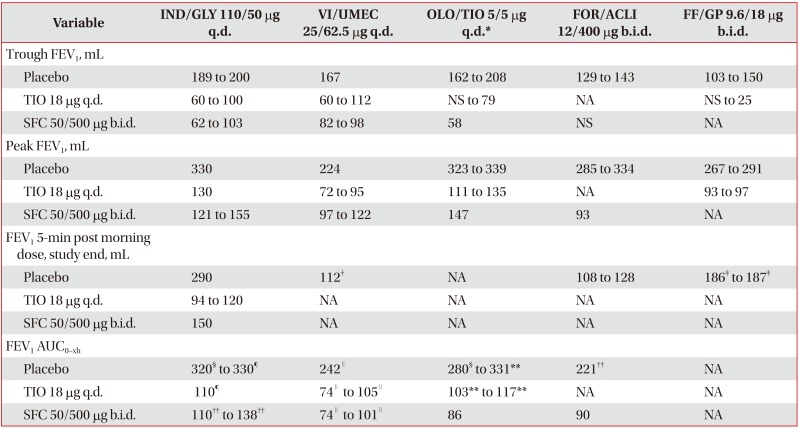
Values are presesnted as minimum and maximum mean LSM treatment difference value from all trials analyzed.
*For OLO/TIO 5/5 µg q.d. studies, TIO 5 µg q.d. used as comparator. †FEV1 15-min post morning dose on day 1. ‡FEV1 5-min post morning dose, day 1. §FEV1 AUC0–24h. ¶FEV1 AUC0–4h. ∥FEV1 AUC0–6h. **FEV1AUC0–3h. ††FEV1 AUC0–12h.
LABA/LAMA: long-acting β2-agonist/long-acting muscarinic antagonist; FDC: fixed-dose combination; TIO: tiotropium; SFC: salmeterol/fluticasone; IND/GLY: indacaterol/glycopyrronium; q.d.: once daily; VI/UMEC: vilanterol/umeclidinium; OLO/TIO: olodaterol/tiotropium; FOR/ACLI: formoterol/aclidinium; b.i.d.: twice daily; FF/GP: formoterol fumarate/glycopyrrolate; FEV1: forced expiratory volume in 1 second; NS: non-significant; NA: not available (no outcomes in any of the trials evaluated); AUC: area under the curve.
2. Dyspnea
Table 4
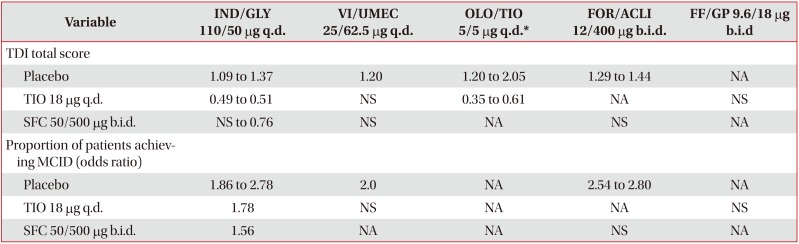
Values are presented as LSM treatment difference, unless otherwise specified. Data expressed as minimum and maximum mean value from all trials analyzed.
*For OLO/TIO 5/5 µg q.d. studies, TIO 5 µg q.d. used as comparator.
LABA/LAMA: long-acting β2-agonist/long-acting muscarinic antagonist; FDC: fixed-dose combination; TIO: tiotropium; SFC: salmeterol/fluticasone; IND/GLY: indacaterol/glycopyrronium; ; q.d.: once daily; VI/UMEC: vilanterol/umeclidinium; OLO/TIO: olodaterol/tiotropium; FOR/ACLI: formoterol/aclidinium; b.i.d.: twice daily; FF/GP: formoterol fumarate/glycopyrrolate; TDI: transition dyspnea index; NA: not available (no outcomes in any of the trials evaluated); TIO: tiotropium; NS: non-significant; SFC: salmeterol/fluticasone; MCID: minimum clinically important difference.
3. Health-related quality of life and rescue medication use
Table 5
Improvement in health-related quality of life and reduction in rescue medication use with LABA/LAMA FDCs versus placebo, TIO and SFC262728313335363840414243454748495153
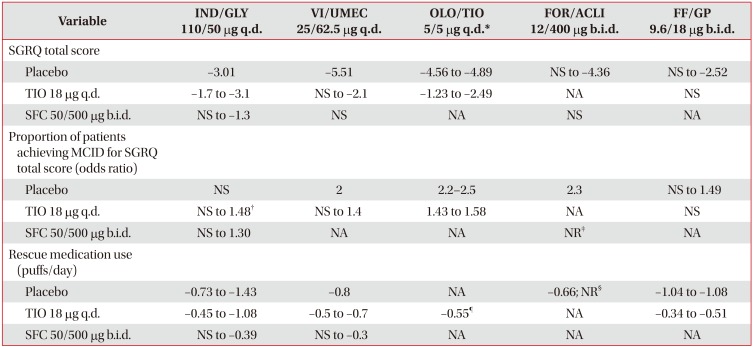
Data expressed as minimum and maximum mean value from all trials analyzed.
*For OLO/TIO 5/5 µg q.d. studies, TIO 5 µg q.d. used as comparator. †Differences were statistically significant at all time points up to Week 52 (at week 64, p=0.051). ‡52.6% patients in FOR/ACLI arm and 55.8% in SFC arm achieved MCID for SGRQ total score. §Significant reductions in the use of rescue medication versus placebo were also observed in the AUGMENT study (puffs/day not reported). ¶Approximate value.
LABA/LAMA: long-acting β2-agonist/long-acting muscarinic antagonist; FDC: fixed-dose combination; TIO: tiotropium; SFC: salmeterol/fluticasone; IND/GLY: indacaterol/glycopyrronium; q.d.: once daily; VI/UMEC: vilanterol/umeclidinium; OLO/TIO: olodaterol/tiotropium; FOR/ACLI: formoterol/aclidinium; b.i.d.: twice daily; FF/GP: formoterol fumarate/glycopyrrolate; SGRQ: St. George's Respiratory Questionnaire; NS: non-significant; NA: not available (no outcomes in any of the trials evaluated); MCID: minimum clinically important difference; NR: not reported (outcomes not reported in required units).
4. COPD exacerbations
1) LABA/LAMA versus placebo or TIO
Table 6
Annualized rate and time to first exacerbation with LABA/LAMA FDCs versus placebo, TIO, and SFC26272829313238404750
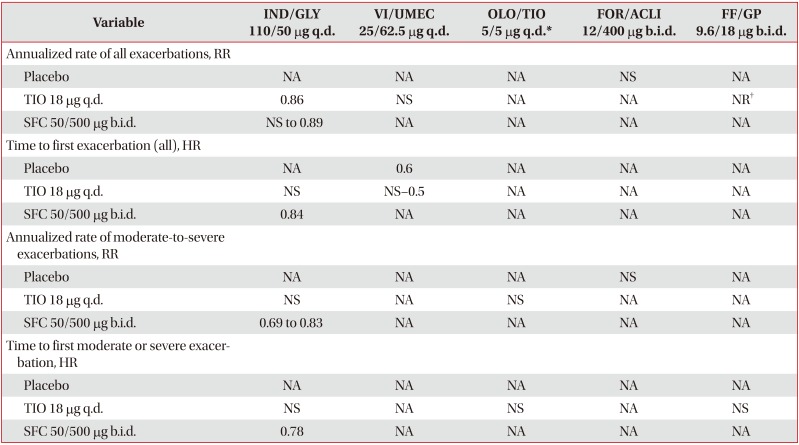
Data expressed as minimum and maximum mean value from all trials analyzed.
*For OLO/TIO 5/5 µg q.d. studies, TIO 5 µg q.d. used as comparator. †23% of patients in FF/GP 9.6/18 µg b.i.d. group and 25.1% in open-label TIO 18 µg q.d. group experienced exacerbation of any severity.
LABA/LAMA: long-acting β2-agonist/long-acting muscarinic antagonist; FDC: fixed-dose combination; TIO: tiotropium; SFC: salmeterol/fluticasone; IND/GLY: indacaterol/glycopyrronium; q.d.: once daily; VI/UMEC: vilanterol/umeclidinium; OLO/TIO: olodaterol/tiotropium; FOR/ACLI: formoterol/aclidinium; b.i.d.: twice daily; FF/GP: formoterol fumarate/glycopyrrolate; RR: rate ratio; NA: not available (no outcomes in any of the trials evaluated); NS: non-significant; NR: not reported (outcomes not reported in required units); HR: hazard ratio.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download