Abstract
Acute exacerbation(s) of chronic obstructive pulmonary disease (AECOPD) tend to be critical and debilitating events leading to poorer outcomes in relation to chronic obstructive pulmonary disease (COPD) treatment modalities, and contribute to a higher and earlier mortality rate in COPD patients. Besides pro-active preventative measures intended to obviate acquisition of AECOPD, early recovery from severe AECOPD is an important issue in determining the long-term prognosis of patients diagnosed with COPD. Updated GOLD guidelines and recently published American Thoracic Society/European Respiratory Society clinical recommendations emphasize the importance of use of pharmacologic treatment including bronchodilators, systemic steroids and/or antibiotics. As a non-pharmacologic strategy to combat the effects of AECOPD, noninvasive ventilation (NIV) is recommended as the treatment of choice as this therapy is thought to be most effective in reducing intubation risk in patients diagnosed with AECOPD with acute respiratory failure. Recently, a few adjunctive modalities, including NIV with helmet and helium-oxygen mixture, have been tried in cases of AECOPD with respiratory failure. As yet, insufficient documentation exists to permit recommendation of this therapy without qualification. Although there are too few findings, as yet, to allow for regular andr routine application of those modalities in AECOPD, there is anecdotal evidence to indicate both mechanical and physiological benefits connected with this therapy. High-flow nasal cannula oxygen therapy is another supportive strategy which serves to improve the symptoms of hypoxic respiratory failure. The therapy also produced improvement in ventilatory variables, and it may be successfully applied in cases of hypercapnic respiratory failure. Extracorporeal carbon dioxide removal has been successfully attempted in cases of adult respiratory distress syndrome, with protective hypercapnic ventilatory strategy. Nowadays, it is reported that it was also effective in reducing intubation in AECOPD with hypercapnic respiratory failure. Despite the apparent need for more supporting evidence, efforts to improve efficacy of NIV have continued unabated. It is anticipated that these efforts will, over time, serve toprogressively decrease the risk of intubation and invasive mechanical ventilation in cases of AECOPD with acute respiratory failure.
Chronic obstructive lung disease is characterized by airflow limitation and chronic airway inflammation and it is expected to be the third leading cause of death in 20301. In the natural course of chronic obstructive pulmonary disease (COPD), acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is a critical event leading to poorer outcomes of COPD. AECOPD is clinically defined as the events of increasing respiratory symptoms including dyspnea, cough, and sputum production, and increased sputum purulence23. It is manifested with the exaggerated airway inflammation and bronchial obstruction.
AECOPD is a trigger for catastrophic cascades in COPD and it is one of the most important risk predictor for subsequent exacerbation, lung function decline, and mortality 45. Severe AECOPD requires hospitalization or visiting the emergency room and it may be associated with acute respiratory failure. One-year mortality of patients with AECOPD requiring noninvasive ventilation (NIV) and intensive care unit (ICU) care is reported to be as high as 28% and 43%, respectively6. Therefore, prevention of AECOPD is one of the most important goal in manging COPD. Once AECOPD develops, early recovery is crucial in determining prognosis of patients with COPD and delayed recovery from exacerbation accelerates decline in forced expiratory volume in 1 second (FEV1)7. Therefore, as well as prevention of AECOPD, efforts to recover from the disaster as soon as possible is required.
In this review, recent updates for management of AECOPD will be discussed with special focus on the patients with acute respiratory failure and life-threatening exacerbation.
Short acting bronchodilators (short-acting inhaled β2-agnosits, with or without short-acting anticholinergics) are recommended as an initial choice to treat an AECOPD8. The short acting bronchodilators can be administered using nebulizer or metered dose inhaler (MDI) with spacer. Even though the emitted dose of drug is larger in nebulizer than MDI plus spacer, relative lung deposition of short acting bronchodilator following inhalation in AECOPD is similar in both delivery systems9. Long-acting bronchodilators also should be applied as soon as possible in AECOPD.
Systemic steroids is a cornerstone of treating AECOPD and it can improve FEV1, oxygenation, shorten recovery time, and hospital stay. Some data suggested the association between response to corticosteroids and blood eosinophil count10. Recently, American Thoracic Society/European Respiratory Society reported updated clinical recommendation for treating AECOPD in terms of six key questions including “Should oral corticosteroids be used to treat ambulatory patients who are having a COPD exacerbation?”8. It recommended a short course of oral corticosteroids even for ambulatory patients with AECOPD.
Although the roles of antibiotics in AECOPD have been debated, antibiotics can be used when patients have clinical sings of bacterial infection such as increased sputum and purulence. In a systemic review of placebo-controlled studies, antibiotics reduced short-term mortality, treatment failure, and sputum purulence10.
AECOPD with respiratory failure can be manifested with hypoxemic respiratory failure and/or ventilatory failure. Supplementary oxygen is essential to improve patient's hypoxemia and it should be titrated as treatment goes on with target saturation of 88%–92% and assessing CO2 retention1112.
If respiratory acidosis progresses despite proper pharmacological and oxygen therapy, ventilatory support should be considered and applied to treat acute respiratory failure. Until now, whereas NIV is regarded as the first choice for ventilatory support in AECOPD, the following some adjunctive strategies with NIV and high-flow nasal cannula (HFNC) oxygen therapy may have limited controlled trials and evidence for in AECOPD. However, recent updated reports may be helpful in managing AECOPD with respiratory failure and show a breakthrough point in current practice.
NIV is preferentially recommended in AECOPD over invasive mechanical ventilation with intubation1213. Trends in mechanical ventilation among patients with AECOPD have been changed. For an example in U.K., all mechanical ventilatory support has been increased14. Initial NIV increased by 15.1% yearly while invasive mechanical ventilation declined by 3.2% as shown in Figure 1 and the trends is predominant as the frequency of administration was increased and in patients without pneumonia14. NIV can reduce intubation risk and complication including ventilatory associated pneumonia. It is also favored in reducing mortality15. The benefits of NIV is achieved by recruitment of collapsed alveoli and improving ventilation-perfusion imbalance resulting in improving oxygenation and respiratory acidosis. Positive ventilation will decrease work of breathing by increasing mean airway pressure and decreasing air trapping. The efficacy of NIV in acute respiratory failure due to AECOPD in terms of improving treatment failure, mortality, and intubation risk is well reviewed in remote and recent meta-analyses. Therefore, for hospitalized patients with acute or acute-on-chronic hypercapnic respiratory failure due to AECOPD, the use of NIV is strongly recommended. General indications for NIV include (1) respiratory acidosis (partial pressure of carbon dioxide [PaCO2] ≥45 mm Hg and pH ≤7.35); (2) severe dyspnea with clinical signs in use of respiratory accessary muscles, paradoxical movement, and retraction of intercostal muscles; and (3) persistent hypoxemia despite oxygen therapy.
When NIV is applied with a face mask, patients frequently suffer from many complications including pressure intolerance, air leak related adverse effects (sleep disruption, reduced ventilation, and eye irritation), skin erosion on nasal bridge, naso-oral airway complications (dryness, nasal congestion, rhinitis, and bleeding), and aerophagia (eructation, flatulence, and abdominal disturbance)16. Because of high complication rate, overall failure of NIV occurs in 16%–30% of patients17. NIV with a helmet was developed to improve performance and reduce complication of NIV with mask. It has advantages in terms of nutrition and hydration through patient access port, lower air leaks, no facial skin lesions, no eye irritation, and independence of the patient's anatomy as shown in Figure 2. However, it has disadvantages for noise, larger dead space, and the risk of claustrophobia17.
Despite the previous controversy on effects of oxygenation and CO2 retention, reduction on intubation rate and mortality, recent studies reported favorable results. For example, in a randomized controlled study in patients with adult respiratory distress syndrome (ARDS), NIV delivered by helmet showed lower mortality and decreased endotracheal intubation (absolute difference –21.4% for mortality and –43.3% for endotracheal intubation)18. Also, a recent meta-analysis of controlled studies for NIV with helmet in patients with acute respiratory failure showed that NIV with a helmet was associated with lower hospital mortality (odds ratio [OR], 0.43; 95% confidence interval [CI], 0.26–0.69; p=0.0005), intubation rate (OR, 0.32; 95% CI, 0.21–0.47; p<0.00001), and complications (OR, 0.6; 95% CI, 0.4–0.92; p=0.02)17. However, until now, trials of NIV with helmet in patients with AECOPD have been rarely performed or tested in small population. In a small randomized controlled study recruited patients with AECOPD, there is no statistical difference in length of ICU stay, ICU mortality, NIV failure, and complications in helmet group and facial mask group19. In a multi-center short-term randomized controlled study for patients with acute hypercapnic respiratory failure, NIV with hemet significantly improved ventilation parameters (pH, PaCO2, partial pressure of oxygen [PaO2]/fraction of inspired oxygen [FiO2], respiration rate), heart rate, and dyspnea score than baseline level and the improved results were similar to those of oronasal mask20. Therefore, helmet may be a valid alternative option to a mask in improving gas exchange and achieving a good tolerance to NIV. However, higher level of positive end-expiratory pressure or pressure support may be required to compendate the larger dead space18.
Another adjunctive treatment for NIV ever tried is the use of helium-oxygen mixture (He/O2). He/O2 has lower density than air/oxygen and it enhances transition to laminar flow and reduce density dependent components of airway resistance. Therefore, hypothetically, it is expected to reduce NIV failure rate when using He/O2 instead of air/oxygen in AECOPD. However, in recent meta-analysis including relevant three randomized controlled trials enrolled a total of 772 patients, He/O2 didn't reduce NIV failure rate and ICU mortality rate compared with air/oxygen21. Nevertheless, NIV with He/O2 was associated with less NIV-related adverse events (OR, 0.56; 95% CI, 0.4–0.8; p=0.001) and a shorter length of ICU stay (difference in means, –1.07 day; 95% CI, –2.14 to –0.004; p=0.049) in hypercapnic COPD exacerbation21.
An extended prospective, randomized, open-label trial in 16 ICUs and six countries was performed to elucidate the efficacy of He/O2 with continuous administration for 72 hours in severe exacerbation of COPD. This trial was closed early due to low global NIV failure rate and the primary outcomes, NIV failure rate and intubation rate, were not different in He/O2 and air/oxygen group. In subgroup analysis for patients failed with NIV, the ventilation duration and length of ICU stay was significantly shorter in He/O2 22. Additionally, primary outcomes seemed to be better in trends and showed better ventilation outcomes for initial three days of treatment without final statistical significance22.
Extracorporeal life support (ECLS) has been rapidly progressed for treating ARDS and expanding its indications including hypercapnic respiratory failure, bride to lung transplantation, earlier use in less severe hypoxemic respiratory failure, resuscitation of donor lungs before transplantation, and bridge to early mobility 23. Extracorporeal CO2 removal (ECCO2R) as a kind of ECLS has been focused on improving hypercapnia during protective ventilation strategy in ARDS. Comparing with conventional extracorporeal membrane oxygenation, ECCO2R presents many advantages including a lower blood flow rate and consequently smaller veno-venous catheters (Table 1). Therefore, ECCO2R has been applied for non-ARDS hypercapnic respiratory failure as case studies or single centered prospective pilot studies23. In a multicenter retrospective randomized controlled study in patients with hypercapnic respiratory failure failing NIV, arterio-venous ECCO2R group avoided intubation risk (10% for intervention group vs. 100% for controlled group) and showed short term and long-term survival24. Another multicenter matched cohort study with historical control in patients with hypercapnic respiratory failure also decreased the risk of intubation to a third25. In the matched cohort study with historical control, when ECCO2R with NIV was applied for hypercapnic patients at risk of NIV failure, respiratory variables such as PaCO2, arterial pH, respiratory rate, and PaO2/FiO2 were significantly improved and cumulative incidence of endotracheal intubation (hazard ratio, 0.27; 95% CI, 0.07–0.98; p=0.047) and hospital mortality (8% vs. 35%, p=0.0347) were significantly lowered in patients applied with NIV and ECCO2R25. Recently, a multicenter case-control study was performed in five European ICUs to evaluate the feasibility and safety of avoiding invasive mechanical ventilation by using ECCO2R in patients with AECOPD and acute hypercapnic respiratory failure refractory to NIV26. Although it was a small study, the primary outcome, intubation risk was avoided in 56% of patients in the ECCO2R group with extracorporeal blood flow of 1.3 L/min. In this trial, major bleeding was developed in 36% of patients which was significantly higher than compared with control (8%)26. Further studies with ECCO2R for prevention of intubation in AECOPD, facilitating extubation, and benefits in stable COPD with chronic hypercapnic failure are ongoing.
Modern ECLS including ECCO2R offers new and promising therapeutic options in patients with COPD in acute on chronic respiratory failure. However, severe complications including hemorrhage and needs for further evidence on the overall efficacy in AECOPD may be obstacles to expanding clinical application of NIV with ECCO2R23. Also, the right indication, timing, and strategy for applying ECCO2R in patients with AECOPD should be further explored27.
HFNC oxygen therapy can deliver a heated and humidified air flow of 20–50 L/min with or without supplementary oxygen and it has been reported to be an effective method to prevent post-extubation respiratory failure and reduce intubation failure rate in acute hypoxemic respiratory failure. Physiologically, HFNC oxygen therapy reduces functional dead space and decrease nasopharyngeal resistance and exerts a positive expiratory pressure effect and increase alveolar recruitment resulting in improving ventilation-perfusion mismatching28. It also contributes to humidify airway mucosa. In patients with stable hypercapnic COPD, the overall physiologic effects can lead to improve respiratory pattern (decreased respiration rate, increased tidal volume, increased airway pressure, decreased work of breathing, and improving hypercapnia, etc.) in a minor flow dependent pattern (Figure 3)2930.
Despite HFNC oxygen therapy showed beneficial effects in acute hypoxemic respiratory failure31, the clinical significance of HFNC oxygen therapy in AECOPD has not been widely studied and the role of HFNC oxygen therapy in hypercapnic respiratory failure may be controversial. Recently, a small randomized controlled cross-over trial to evaluate the physiologic effects of titrated oxygen via HFNC compared with standard nasal prongs in Asian patients with AECOPD by measuring transcutaneous CO2 tension (PtCO2)32. The difference in PtCO2 adjusted for time zero was significantly lower after 30 minutes for HFNC compared with standard nasal prong (–1.4 mm Hg) (95% CI, –2.2 to –0.6, p=0.001) while there was no difference in SpO2 (p=0.96).
As well as pharmacologic treatment, NIV is recommended as a beneficial strategy to reduce intubation risk in AECOPD with acute respiratory failure. Overcoming shortness of conventional NIV, some adjunctive modalities including NIV with helmet and helium-oxygen mixture have been tried. NIV with a helmet was noninferior to conventional facial mask in reducing intubation risk while avoiding commo complications of mask. Until now, NIV with helium-oxygen mixture didn't show any superiority compare to air-oxygen ventilation in terms of NIV failure rate and ICU mortality. HFNC oxygen therapy is another supportive strategy improving hypoxic respiratory failure and it also showed improvement in ventilatory variables and can be applicable in hypercapnic respiratory failure. As ECCO2R has been successfully applied in ARDS with protective hypercapnic ventilatory strategy, some reports showed effectiveness in reducing intubation in AECOPD with hypercapnic respiratory failure adjunctive with NIV. Currently, despite the shortness of evidence, efforts to improve efficacy of NIV will be continued and it may be expected to decrease the risk of intubation and invasive mechanical ventilation in AECOPD with acute respiratory failure.
Figures and Tables
 | Figure 1Trends in initial ventilation. IMV: invasive mechanical ventilation; MV: mechanical ventilation; NIV: noninvasive ventilation. Adapted from Stefan et al. Chest 2015;147:959-68, with permission of Elsevier14. |
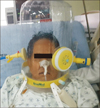 | Figure 2A patient with acute respiratory failure supported by noninvasive ventilation with a helmet (The patient provided written informed consent). |
 | Figure 3The effect of high-flow nasal cannula oxygen therapy on changes in mean airway pressure (A) and tidal volume (B) in patients with stable hypercapnic chronic obstructive pulmonary disease. nCPAP: nasal continuous positive airway pressure; nBiPAP: nasal bi-level positive airway pressure. Adapted from Braunlich et al. Int J Chron Obstruct Pulmon Dis 2016;11:1077-85, according to the Creative Commons license Dove Medical Press30. |
Table 1
Characteristics of ECMO and ECCO2R

References
1. European Respiratory Society. European Respiratory Society's white book. Sheffield: European Respiratory Society;2016.
2. Yoon HK, Park YB, Rhee CK, Lee JH, Oh YM. Committee of the Korean COPD Guideline 2014. Summary of the chronic obstructive pulmonary disease clinical practice guideline revised in 2014 by the Korean Academy of Tuberculosis and Respiratory Disease. Tuberc Respir Dis. 2017; 80:230–240.

3. Singh D. Pharmacological treatment for COPD; GOLD 2017 changes direction. Br J Clin Pharmacol. 2017; 83:935–937.

4. Zhou A, Zhou Z, Zhao Y, Chen P. The recent advances of phenotypes in acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2017; 12:1009–1018.

5. Hillas G, Perlikos F, Tzanakis N. Acute exacerbation of COPD: is it the “stroke of the lungs”? Int J Chron Obstruct Pulmon Dis. 2016; 11:1579–1586.
6. Ko FW, Chan KP, Hui DS, Goddard JR, Shaw JG, Reid DW, et al. Acute exacerbation of COPD. Respirology. 2016; 21:1152–1165.

7. Donaldson GC, Law M, Kowlessar B, Singh R, Brill SE, Allinson JP, et al. Impact of prolonged exacerbation recovery in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015; 192:943–950.

8. Wedzicha JA, Miravitlles M, Hurst JR, Calverley PM, Albert RK, Anzueto A, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017; 49:1600791.
9. Mazhar SH, Ismail NE, Newton DA, Chrystyn H. Relative lung deposition of salbutamol following inhalation from a spacer and a Sidestream jet nebulizer following an acute exacerbation. Br J Clin Pharmacol. 2008; 65:334–337.

10. Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012; 186:48–55.
11. Austin MA, Wills KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010; 341:c5462.

12. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol. 2017; 53:128–149.

13. Seol YM, Park YE, Kim SR, Lee JH, Lee SJ, Kim KU, et al. Application of noninvasive positive pressure ventilation in patients with respiratory failure. Tuberc Respir Dis. 2006; 61:26–33.

14. Stefan MS, Shieh MS, Pekow PS, Hill N, Rothberg MB, Lindenauer PK. Trends in mechanical ventilation among patients hospitalized with acute exacerbations of COPD in the United States, 2001 to 2011. Chest. 2015; 147:959–968.

15. Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017; 7:CD004104.

16. Esquinas Rodriguez AM, Papadakos PJ, Carron M, Cosentini R, Chiumello D. Clinical review: helmet and non-invasive mechanical ventilation in critically ill patients. Crit Care. 2013; 17:223.

17. Liu Q, Gao Y, Chen R, Cheng Z. Noninvasive ventilation with helmet versus control strategy in patients with acute respiratory failure: a systematic review and meta-analysis of controlled studies. Crit Care. 2016; 20:265.

18. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016; 315:2435–2441.
19. Ozlem CG, Ali A, Fatma U, Mehtap T, Saziye S. Comparison of helmet and facial mask during noninvasive ventilation in patients with acute exacerbation of chronic obstructive pulmonary disease: a randomized controlled study. Turk J Med Sci. 2015; 45:600–606.
20. Pisani L, Mega C, Vaschetto R, Bellone A, Scala R, Cosentini R, et al. Oronasal mask versus helmet in acute hypercapnic respiratory failure. Eur Respir J. 2015; 45:691–699.

21. Abroug F, Ouanes-Besbes L, Hammouda Z, Benabidallah S, Dachraoui F, Ouanes I, et al. Noninvasive ventilation with helium-oxygen mixture in hypercapnic COPD exacerbation: aggregate meta-analysis of randomized controlled trials. Ann Intensive Care. 2017; 7:59.

22. Jolliet P, Ouanes-Besbes L, Abroug F, Ben Khelil J, Besbes M, Garnero A, et al. A multicenter randomized trial assessing the efficacy of helium/oxygen in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017; 195:871–880.

23. Bhatt N, Osborn E. Extracorporeal gas exchange: the expanding role of extracorporeal support in respiratory failure. Clin Chest Med. 2016; 37:765–780.
24. Kluge S, Braune SA, Engel M, Nierhaus A, Frings D, Ebelt H, et al. Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med. 2012; 38:1632–1639.

25. Del Sorbo L, Pisani L, Filippini C, Fanelli V, Fasano L, Terragni P, et al. Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med. 2015; 43:120–127.
26. Braune S, Sieweke A, Brettner F, Staudinger T, Joannidis M, Verbrugge S, et al. The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case-control study. Intensive Care Med. 2016; 42:1437–1444.
27. Del Sorbo L, Fan E, Nava S, Ranieri VM. ECCO2R in COPD exacerbation only for the right patients and with the right strategy. Intensive Care Med. 2016; 42:1830–1831.

28. Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016; 61:529–541.

29. Silva Santos P, Esquinas AM. The use of high-flow nasal oxygen in COPD patients. Int J Chron Obstruct Pulmon Dis. 2016; 11:2259–2260.

30. Braunlich J, Kohler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016; 11:1077–1085.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download