Introduction of Assessment Indicators of Lung Cancer Management Proposed by Health Insurance Review and Assessment Service of Korea
1. Organization of lung cancer specialists
This indicator is to assess the composition of the medical staff involved in the lung cancer care in the institutions to be evaluated (
Table 1). It aims to improve the coordination and increase opportunities from various perspectives for patients' care. It is fulfilled when the staffs were consisted of at least one member of the pulmonary medicine, hemato-oncology, thoracic surgery, pathology, radiation oncology, radiology, and nuclear medicine.
Table 1
Appropriateness indicators of lung cancer management by HIRA of Korea
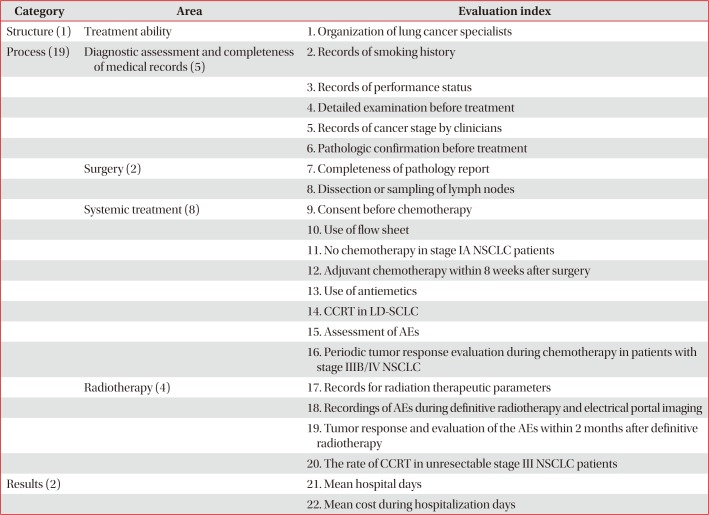
|
Category |
Area |
Evaluation index |
|
Structure (1) |
Treatment ability |
1. Organization of lung cancer specialists |
|
Process (19) |
Diagnostic assessment and completeness of medical records (5) |
2. Records of smoking history |
|
|
3. Records of performance status |
|
|
4. Detailed examination before treatment |
|
|
5. Records of cancer stage by clinicians |
|
|
6. Pathologic confirmation before treatment |
|
Surgery (2) |
7. Completeness of pathology report |
|
|
8. Dissection or sampling of lymph nodes |
|
Systemic treatment (8) |
9. Consent before chemotherapy |
|
|
10. Use of flow sheet |
|
|
11. No chemotherapy in stage IA NSCLC patients |
|
|
12. Adjuvant chemotherapy within 8 weeks after surgery |
|
|
13. Use of antiemetics |
|
|
14. CCRT in LD-SCLC |
|
|
15. Assessment of AEs |
|
|
16. Periodic tumor response evaluation during chemotherapy in patients with stage IIIB/IV NSCLC |
|
Radiotherapy (4) |
17. Records for radiation therapeutic parameters |
|
|
18. Recordings of AEs during definitive radiotherapy and electrical portal imaging |
|
|
19. Tumor response and evaluation of the AEs within 2 months after definitive radiotherapy |
|
|
20. The rate of CCRT in unresectable stage III NSCLC patients |
|
Results (2) |
|
21. Mean hospital days |
|
|
22. Mean cost during hospitalization days |

2. Records of smoking history
This indicator is for the physician's written record on smoking and pack-years (PYs) before treatment. The ratio of smoking record of patients who is treated with lung cancer is calculated as follows: Number of patients with smoking history/Number of patients treated with lung cancer (surgery, chemotherapy, or radiation therapy)×100.
Smoking is not only a major risk factor for lung cancer, but PY is also useful for predicting postoperative complications related to decreased lung function and mucociliary clearance. In addition, preliminary information on the PY of the patient before surgery is important to plan the treatment modality including surgery, so it is important to identify and record the smoking history and PY before treatment.
3. Records of performance status
This is a measure of the proportion of patients who have an assessment of their performance status (PS) before treatment before surgery, chemotherapy, or radiation therapy for lung cancer. The ratio of performance status record is calculated as follows: Number of patients with a record of performance status before treatment/Number of patients treated with lung cancer (surgery, chemotherapy, or radiation therapy)×100.
This is admitted when the performance status was recorded by the medical staff before the treatment modality, including surgery for pediatric wedge resection, chemotherapy, chemotherapy, radiation therapy, chemotherapy combined with chemotherapy (CCRT) using the Eastern Cooperative Oncology Group (ECOG), World Health Organization (WHO), or Karnofsky scoring system described in
Table 2.
Table 2
ECOG/WHO scoring system and Karnofsky PS status scale
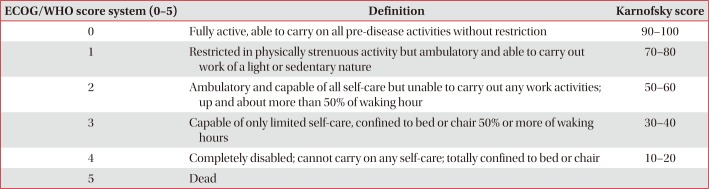
|
ECOG/WHO score system (0–5) |
Definition |
Karnofsky score |
|
0 |
Fully active, able to carry on all pre-disease activities without restriction |
90–100 |
|
1 |
Restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature |
70–80 |
|
2 |
Ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hour |
50–60 |
|
3 |
Capable of only limited self-care, confined to bed or chair 50% or more of waking hours |
30–40 |
|
4 |
Completely disabled; cannot carry on any self-care; totally confined to bed or chair |
10–20 |
|
5 |
Dead |
|

4. Detailed examination before treatment
This index refers to the percentage of patients who underwent close-up screening examination for lung cancer before treatment including surgery, chemotherapy, or radiation therapy (
Table 3). These include (1) chest computed tomography (CT) that includes upper abdomen and adrenal glands; (2) positron emission tomography (PET)-CT/PET in the stage IB–III non-small cell lung cancer (NSCLC) patients; (3) brain CT or brain magnetic resonance image (MRI) in the limited disease (LD)–small cell lung cancer (SCLC) and stage II-III NSCLC patients; (4) lung function test in the patients who underwent lung cancer surgery and definitive radiation therapy; (5) mediastinoscopy and endobronchial ultrasound-guided transbronchial needle aspiration or video-assisted thoracoscopic surgery in N2 NSCLC patients (excluding stage IV); and (6) epidermal growth factor receptor (
EGFR) mutation test (monitoring) in the stage IV lung adenocarcinoma patients
1.
Table 3
Radiologic tests and their evaluation subjects in patients with lung cancer

|
Radiologic test |
Evaluation subject |
|
Chest CT including upper abdomen/adrenal glands (within 60 days from beginning of initial treatment) |
All patients diagnosed as lung cancer |
|
PET or PET-CT |
Stage IB–III NSCLC |
|
Brain CT or MRI |
LD-SCLC |
|
Stage II, III NSCLC |

Prior to treatment, it is essential to perform highly sensitive tests to determine the status of patients and the lung cancer stage
23. (1) Chest CT is an indispensable tool for assessment of tumor size, location, and involvement with surrounding organs, and lymph node
4. (2) PET-CT or PET is useful for determining metastatic disease
2. (3) Brain CT or brain MRI is useful for confirming the presence of brain metastases
56. (4) Pulmonary function tests (forced expiratory volume in 1 second [FEV
1], diffusion lung capacity for carbon monoxide) are helpful in assessing the risk associated with surgery or radical radiotherapy
789. (5) Mediastinoscopy, endobronchial ultrasound, and mediastinal lymph node dissection should be performed prior to treatment for mediastinal staging if N2 stage is suspected because mediastinal stage has a significant effect on treatment decisions and prognosis
101112. (6) In NSCLC patients who are not candidate of curative therapy,
EGFR mutation test should be performed because EGFR tyrosine kinase inhibitors improve response rates, time to progression, and overall survival compared with systemic chemotherapy in patients harboring activating
EGFR mutation
1314. In case all the relevant tests are been carried out, this Indicator is considered to fulfill the requirement. When the examinations were carried out by other institute(s), including an external agency, it would be recognized when there is corresponding inspection films or result sheets. Regarding the chest CT, it should be taken within 60 days from the starting day of treatment and should include epigastrium and adrenal gland. If there is a written description on the epigastrium and adrenal gland, it is acknowledged and in this case, the reading findings for the upper abdomen should be provided. In case that chest CT scan does not include epigastric and adrenal glands, it would be acknowledged when PET-CT/PET or abdominal CT scan identifies these regions. Regarding pulmonary function tests, FEV
1 alone is accepted when FEV
1 is equal or more than 80% of reference value and there are no abnormal symptoms such as dyspnea.
EGFR mutation test is recognized if it is confirmed before the start of treatment and in case of radiation therapy on the metastatic lesion, the test after radiotherapy would be accepted.
However, when the patient who does have any lung cancer related diagnosis underwent emergency surgery, the patient refused examination except chest CT, brain CT/brain MRI, and pulmonary function test, and patient status is too poor to take examination, it is excluded from evaluation. In addition, if the EGFR mutation test is not performed due to insufficient specimen, this case shall be excluded from the evaluation by including the reason for exclusion such as the pathologic or the doctor's medical records.
5. Records of cancer stage by clinicians
Clinical cancer stage is assessed by the ratio of complete records of pretreatment stage by clinicians to all lung cancer patients underwent any treatment modality. For the clinical staging, clinicians should describe the records before treatment using TNM staging system according to the American Joint Committee on Cancer (7th edition) for NSCLC and a two-stage classification (LD or extensive disease [ED]) or TNM staging system for SCLC. In patients who underwent nonsurgical treatment, pretreatment staging should be reported by one of clinicians including pulmonologist, oncologist, thoracic surgeon, and radiation oncologist. In patients who underwent surgical treatment, thoracic surgeon could document complete records of clinical stage within 28 days after surgery. In cases of neoadjuvant treatment prior to surgery, clinicians (pulmonologist, oncologist, thoracic surgeon or radiation oncologist) must document clinical stage before surgery. When the institution shares staging records among clinicians or the trainee documents the clinical cancer stage in a teaching hospital, the final signature by a corresponding specialist is accepted for the evaluation.
6. Pathologic confirmation before treatment
Pretreatment confirmative diagnosis is evaluated by the proportion of patients with pretreatment pathological diagnosis out of total lung cancer patients who underwent nonsurgical treatment. Referral patients who have only pathology reports from outside hospital are also included. Stage IV lung cancer patients with palliative radiation therapy are excluded.
7. Completeness of pathology report
Completeness of surgical pathology report is assessed by the ratio of complete pathology reports to all surgically resected lung cancer specimens. Surgical specimens requested to the referral hospitals for pathologic examination are also included. Every complete pathology report should document all essential pathological data that will affect patient's treatment and outcome and pathologists signature. Essential pathologic data include tumor site, tumor size, histologic tumor type according to the WHO classification, visceral pleural invasion, lymphovascular invasion, perineural invasion, pathologic staging, lymph node status, surgical resection margin status, and nonneoplastic pathologic pulmonary findings such as interstitial pulmonary fibrosis and tuberculosis. For the pathologic staging, each T and N factor must be clearly defined (e.g., pT2N2 and pT2Nx). Lymph node state should be reported as the number of lymph nodes involved and the total number of lymph nodes submitted. Despite of no residual tumor, lymph node status and nonneoplastic pulmonary findings are required. The reason for missing pathologic parameters should be documented for the complete report.
8. Dissection or sampling of lymph nodes
This index is defined as the proportion of patients underwent lymph nodes dissection or sampling out of all surgically resected lung cancer patients. Systemic mediastinal lymph node dissection or sampling is recommend for complete resection because it discovered pathologic N2 involvement in 24% of patients with clinical stage I and II NSCLC
15. For patients undergoing sublobar resection (segmentectomy or wedge resection), the appropriate N1 and N2 lymph node station should be sampled unless not technically feasible
16. In patients with N2 disease, more than three ipsilateral mediastinal lymph node stations should be dissected during surgery
1517. N2 disease is based upon the clinical cancer stage by thoracic surgeon. The patients with (1) postoperative stage IIIB or IV, (2) limited cardiopulmonary function (e.g., chronic obstructive pulmonary disease), (3) inaccessible lymph node less than three stations due to prior surgery, (4) documented reasons for missing lymph node dissection or sampling, (5) pure adenocarcinoma in situ or minimally invasive adenocarcinoma, and (6) ground glass opacity on chest CT are excluded.
9. Consent before chemotherapy
This Indicator refers to the percentage of patients who have provided a written statement on the chemotherapy to the patient or family member who received chemotherapy and who have a consent record. The ratio of patient (or family) agreement for chemotherapy is calculated as follows: Number of patients with a record of agreement with the description of chemotherapy/Number of patients treated with chemotherapy×100. The patient and physician should discuss the positive effects and possible risks of the chemotherapy and the discussion should include the evidence of treatment, treatment related complications and patient's choice according to the prognosis. This indicator is admitted when there is a written consent signed by a doctor who provides sufficient explanation for the chemotherapy and if the nurse is on the job, the doctor's final signature is required. The purpose of the chemotherapy, the type of chemotherapeutic agents, the time or duration of the chemotherapy, and the main side effects should be included. Finally, a written consent form is issued prior to the initiation of chemotherapy, and a new consent form must be filled out whenever the regimen is changed.
10. Use of flow sheet
This index is the percentage of lung cancer patients who received chemotherapy using a flow sheet. The ratio of patients using flow sheet among lung cancer patients who received chemotherapy is calculated as follows: Number of patients using flow sheet/Number of patients treated with chemotherapy×100.
By using flow sheet on the chemotherapy, accurate recording of chemotherapy and monitoring patient status can facilitate patient status assessment and patient education. This flow sheet should include both oral and parenteral anticancer drugs, excluding oral target drugs, and include five main items such as the purpose of chemotherapy, type of chemotherapy, number of cycles, duration, blood counts such as white blood count or absolute neutrophil count, hemoglobin, and platelet. However, patients with institutional review board (IRB)-approved clinical trials are excluded from the evaluation.
11. No chemotherapy in stage IA NSCLC patients
This index refers to the percent of the stage IA lung cancer patients who had not received adjuvant chemotherapy, and it reflects that the adjuvant chemotherapy is not recommended for the stage IA patients. The ratio of patients who do not receive chemotherapy (stage 1A) is as follows: Number of patients who do not receive adjuvant chemotherapy/Number of patients who underwent resection with lung cancer (stage 1A)×100.
In the cases who did not receive prior therapy, the parameter is evaluated for the cancer staging recorded by the thoracic surgeon after surgery, and the cases who have undergone preoperative therapy, it is evaluated based on the clinical cancer stage recorded by a specialist who is in charge of the pretreatment. However, patients with IRB-approved clinical trials are excluded from the evaluation.
12. Adjuvant chemotherapy within 8 weeks after surgery
This indicator refers to the percentage of patients who underwent first adjuvant chemotherapy within 8 weeks of the last therapeutic intervention among patients who underwent surgery for stage II/IIIA of NSCLC (<70 years old, ECOG 0–1). The ratio of adjuvant chemotherapy within 8 weeks after surgery is calculated as follows: Number of patients who underwent adjuvant chemotherapy within 8 weeks after surgery/Number of patients who underwent surgery for stage II/IIIA of NSCLC (<70 years old, ECOG 0–1)×100.
Stage II/IIIA patients with NSCLC are required to adjuvant chemotherapy after surgery and it is recommended to perform adjuvant chemotherapy within 8 weeks considering the period of recovery from surgery and surgical complications. Exclusion criteria of this indicator (the ratio of adjuvant chemotherapy within 8 weeks after surgery) are as follows: (1) patients who were transferred to other institutions within 8 weeks after surgery or died, (2) patients who underwent preoperative therapy, (3) patients who underwent postoperative chemoradiotherapy or only palliative therapy, (4) patients who scheduled postoperative radiation therapy for adjuvant therapy, (5) patients with IRB-approved clinical trials.
Regardless of dosage regimen, all administered (oral or parenteral) chemotherapeutic agents are included in this Indicator. Cancer staging is based on the following criteria: (1) clinical cancer stage recorded by physician before neoadjuvant chemotherapy, (2) cancer stage recorded by thoracic surgeon after surgery when the patients did not receive neoadjuvant chemotherapy. PS is based on the records that were evaluated before the start of adjuvant chemotherapy.
13. Use of antiemetics
This index is the proportion of patients provided with a serotonin antagonist among those who received moderate-to-moderate risk emetogenic chemotherapeutic agents. The patients who underwent emetogenic chemotherapy of moderate to high risk may suffer nausea and vomiting at least 4 days and should be protected during periods of risk. It will be evaluated the percentage of the patients who provided antiemetic agents before administration of chemotherapeutic agents with moderate to severe nausea and vomiting. However, patients with IRB-approved clinical trials are excluded.
14. CCRT for patients with LD-SCLC
This Indicator is the rate of patients who underwent concurrent CCRT in LD-SCLC. The PS is based on the records that were evaluated before the start of CCRT and the score of the PS should be 0–2
18. Radiation therapy should be given when chemotherapy begins one to three cycles, and the combined use of radiotherapy and chemotherapy should be performed within 1 day
1920.
15. Assessment of adverse effects
This is an indicator for the percentage of cases who received chemotherapy for lung cancer and had an assessment of the side effects of chemotherapy before each treatment cycle. Assessment of adverse reactions to chemotherapeutic agents such as non-hematologic toxicity (nausea/vomiting, diarrhea, systemic asthenia, skin rash, peripheral neuropathy, etc.), nephrotoxicity, hepatotoxicity and bone marrow suppression are recommended before administration of chemotherapy. However, cases that do complete one cycle after the onset of chemotherapy and IRB-approved clinical trials are excluded. Regardless of oral or parenteral regimens, all assessments of the adverse effect should be included and approved by the physician if the anticancer drug side-effects are listed on the medical record, including flow sheet, before each cycle. When evaluating side effects, one or more side effects should be indicated and if there are no side effects, it should be indicated as “None.” If oral anticancer drugs are administered alone, side effects should be evaluated every cycle within the first 3 months of administration, and every two to three cycles or every 2 to 3 months after 3 months of administration.
16. Periodic tumor response evaluation during chemotherapy in patients with stage IIIB/IV NSCLC
Physician should record periodic tumor response evaluation during chemotherapy in patients with NSCLC of stage IIIB/IV. They should describe tumor response as complete remission (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to WHO or Response Evaluation Criteria In Solid Tumor (RECIST) criteria. Tumor response assessment should be done every two to three cycles or every 2 to 3 months. The lung cancer stage is based on the clinical cancer stage recorded by the specialist before the initiation of chemotherapy. Tumor response assessment methods are used by imaging modalities such as chest X-ray, CT, MRI, and PET-CT, etc.
17. Records for radiation therapeutic parameters
There should be a record of the therapeutic radiation parameters as follows: total radiation dose, radiation dose per fraction (or a number of the fraction), and treatment site.
18. Recordings of adverse events during definitive radiotherapy and electrical portal imaging
The radiation oncologists usually interview patients who receive radiotherapy once a week in the course of treatment to evaluate the side effects of radiotherapy. This Indicator is used to evaluate whether the side effects and the accuracy of the radiation field placement using electronic portal imaging devices (EPID). The radiation oncologist must record once a week, and a delay of 1–2 days due to holidays or other reasons is possible. If there is EPID checklist in the picture archiving and communication system, the documents accepted as chart records.
19. Tumor response and evaluation of the adverse events within 2 months after definitive radiotherapy
Radiation oncologists must record and should describe tumor response as CR, PR, SD, or PD according to WHO or RECIST criteria. If there is no side effect, it should be recorded as “None.”
20. The rate of CCRT in unresectable stage III NSCLC patients
This indicator is the rate of patients who underwent CCRT in unresectable stage III NSCLC. Radiotherapy should be performed on the thoracic lesion and should be performed on the same day as chemotherapy
2122. The PS is based on the records that were evaluated before the start of CCRT and the score of the PS should be 0–1
23. The age of the patient should be less than 70 years.
21. Mean hospital days
This index refers to the average length of hospital stay for patients who underwent surgery for lung cancer. The cases where the number of hospitalization day exceed the upper value or fall below the lower value are excluded and the formula is as follows.
Upper limit=X > [Q3+2.5|Q3−Q1|], Lower limit=X < [Q1−2.5|Q3−Q1|]
X: total medical expenses or length of hospitalization days, Q1: 1st quartile, Q3: 3rd quartile
22. Mean cost during hospitalization days
This indicator is the average cost of patients who are hospitalized for lung cancer surgery. This index also excludes the cases whose hospitalization days are extremely high or low and therefore exceed the upper limit or fall below the lower limit. The definitions for the upper and lower values are the same as those described in the indicator 21.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download