Abstract
Background
Men with chronic obstructive pulmonary disease, have reduced endogenous testosterone levels, but the relationship between pulmonary function and endogenous testosterone levels, is inconsistent. Testicular volume is a known indicator of endogenous testosterone levels, male fertility, and male potency. In the present study, the authors investigated the relationship, between testicular volume and lung function.
Methods
One hundred and eighty-one South Korean men age 40-70, hospitalized for urological surgery, were retrospectively enrolled, irrespective of the presence of respiratory disease. Study subjects underwent pulmonary function testing, prior to procedures, and testicular volumes were measured by orchidometry. Testosterone levels of patients in blood samples collected between 7 AM and 11 AM, were measured by a direct chemiluminescent immunoassay.
Results
The 181 study subjects were divided into two groups, by testicular volume (≥35 mL vs. <35 mL), the larger testes group, had better lung functions (forced vital capacity [FVC]: 3.87±0.65 L vs. 3.66±0.65 L, p=0.037; forced expiratory volume in 1 second [FEV1]: 2.92±0.57 L vs. 2.65±0.61 L, p=0.002; FVC % predicted: 98.2±15.2% vs. 93.8±13.1%, p=0.040; FEV1 % predicted: 105.4±19.5% vs. 95.9±21.2%, p=0.002). In addition, the proportion of patients with a FEV1/FVC of <70%, was lower in the larger testes group. Univariate analysis conducted using linear regression models, revealed that testicular volume was correlated with FVC (r=0.162, p=0.029), FEV1 (r=0.218, p=0.003), FEV1/FVC (r=0.149, p=0.046), and FEV1 % predicted (r=0.178, p=0.017), and multivariate analysis using linear regression models, revealed that testicular volume was a significant predictive factor for FEV1 % predicted (β=0.159, p=0.041).
Chronic obstructive pulmonary disease (COPD) is now considered a systemic disease that affects organs beyond the lungs and airways. The disease is characterized by irreversible airflow obstruction and progressive weight loss, especially loss of lean body mass, which is associated with skeletal muscle dysfunction1. Reduced testosterone levels and hypogonadism have been described in COPD2. In adult men, this reduction is modest, but a progressive decline in testosterone production starts between the fourth and sixth decades of life34. This decline is associated with a simultaneous increase of sex hormone-binding globulin (SHBG) levels, and thus, bioavailable testosterone may decline more rapidly than total testosterone5.
Several studies have shown that middle aged and elderly COPD patients may develop hypogonadism, and the prevalence of hypogonadism in men with COPD can range from 22% to 69%. In addition, hypogonadism has been associated with several other systemic manifestations including osteoporosis, depression, and muscle weakness2678. However, information regarding the relationship between pulmonary function and endogenous testosterone levels in general populations is inconsistent5679. Discrepancies can be mainly attributed to small sample sizes, different patient selection criteria, and circadian variations in testosterone levels. Early morning testosterone levels in young males are, on average, 50% higher than afternoon levels, and reference ranges have usually been derived using morning specimens. Furthermore, testosterone levels can fluctuate substantially on a day-to-day basis. It has been recommended that androgen status should be assessed using more than one measurement10.
Testicular volume has been shown to well represent endogenous testosterone levels, and thus, is considered as an indicator of endogenous testosterone level111213141516, fertility, and potency. Furthermore, unlike testosterone levels, testicular volumes do not fluctuate markedly, and in fact, are relatively stable. Testicular volumes have been found to vary widely between individuals in large-scale studies111217. Furthermore, like lung function18, testicular volume decreases with aging1920. Therefore, in the present study, we have investigated the relationship between testicular volume and pulmonary function.
One hundred and eighty-one South Korean male patients (aged 40–70 years) hospitalized for urological surgery between April 2014 and March 2016 at a single tertiary academic center were retrospectively reviewed irrespective of the presence of respiratory disease. Testosterone levels of all the patients in blood samples collected between 7 AM and 11 AM were measured by a direct chemiluminescent immunoassay (Bayer ADVIA Centaur; assay range, 10–1,500 ng/dL). Regarding smoking histories, “never-smokers” were defined as those who had smoked on average <1 cigarette/day for <6 months or had never smoked. For ever-smokers, pack-years were calculated to quantify tobacco use; 1-pack-year was equivalent to smoking an average of 20 cigarettes per day for 1 year.
Patients with conditions known to strongly influence testicular volume were excluded from the study. Testicular volumes were measured by an experienced urologist using an orchidometry.
Relationships between the variables were analyzed using Pearson's linear correlation. Univariate and multivariate analyses were performed using linear regression modelling to identify independent predictors of lung factor. The Student's t test and the chi-square test were used to compare the variables of the two study groups divided by testicular volume (≥35 mL vs. <35 mL).
The analysis was performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA), and p-values of <0.05 were considered statistically significant.
The characteristics of the 181 study subjects are summarized in Table 1.
Univariate analysis using linear regression models showed that testicular volume was correlated with FVC (r=0.162, p=0.029), FEV1 (r=0.218, p=0.003), FEV1/FVC (r=0.149, p=0.046), and FEV1 % predicted (r=0.178, p=0.017) (Table 2). Multivariate analysis using linear regression models showed that testicular volume significantly predicted FEV1 % predicted (β=0.159, p=0.041) (Table 3).
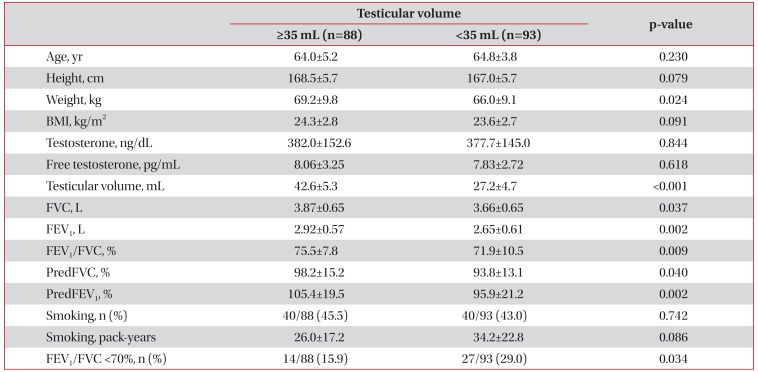
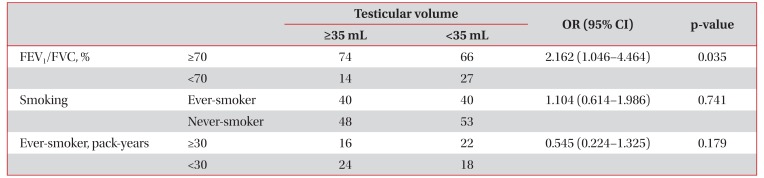
The larger testes group (n=88) had better lung functions than the smaller testes group (n=93) (FVC: 3.87±0.65 L vs. 3.66±0.65 L, p=0.037; FEV1: 2.92±0.57 L vs. 2.65±0.61 L, p=0.002; FEV1/FVC: 75.5±7.8% vs. 71.9±10.5%, p=0.009; FVC % predicted: 98.2±15.2% vs. 93.8±13.1%, p=0.040; FEV1 % predicted: 105.4±19.5% vs. 95.9±21.2%, p=0.002) (Table 4). Furthermore, the percentage of patients with a FEV1/FVC of <70% was lower in the larger testes group (15.9% [14/88] vs. 29.0% [27/93], p=0.034) (Table 4).
Out of the 181 study subjects, 80 (44.2%) were ever-smokers, and the mean smoking exposure of smokers was 30.2±20.5 pack-years (Table 1). Univariate analysis using linear regression models showed that testicular volume was not correlated with smoking (r=−0.094, p=0.216) (Table 2). When patients were divided into two groups according to testicular volume (testicular volume, ≥35 mL vs. <35 mL), proportions of eversmokers and mean smoking exposure of ever-smokers were not different between the two groups (Table 4).
The odds ratio of ever-smokers in the larger versus the smaller testes group was 1.104, which was not statistically significant (95% confidence interval [CI], 0.614–1.986). However, the odds ratio of having a FEV1/FVC ≥70% in the larger testes group versus the smaller testes group was 2.162, which was significant (95% CI, 1.046–4.464) (Table 5).
This is the first study undertaken to investigate whether a relation exists between testicular volume and pulmonary function. It shows testicular volume is positively and independently associated with better lung functions. Furthermore, the proportion of patients with a FEV1/FVC <70% was lower in the larger testes group, and multivariate analysis using linear regression models showed that testicular volume was a significant predictive factor for FEV1 % predicted independently of smoking.
A number of chronic illnesses, including COPD, can cause hypogonadism23. The mechanism of hypogonadism in COPD is multifactorial, and depends on aging, smoking, obesity, systemic inflammation, chronic disease itself, hypoxemia, hypercapnia, and the taking of glucocorticoids24. COPD can affect the hypothalamic-pituitary-testicular axis at multiple levels. In one study, approximately 75% of men with COPD had a low serum testosterone level, a low or below-normal gonadotropin level7. Reduced lung function and decline in lung function are associated with morbidity and mortality. However, the effects of sex hormones on lung function are not well understood. In a previous cross-sectional study conducted on 2,197 men, total and free testosterone levels were found to be positively and independently associated with FVC and FEV1, and men with severe pulmonary obstruction had lower free testosterone levels9. However, another study found no relationship between free testosterone level and FEV1, residual volume, or total lung capacity7. In present study, no relationship was observed between testosterone level and lung function. These discrepancies can be attributed to small sample sizes, differences in patient selection, and the inconsistent testosterone levels.
In an autopsy study, the total volume of Leydig cells in the testes of men with a history of chronic bronchitis and emphysema of at least 15 years' duration, and with morphological evidence of the cardiopulmonary effects of hypoxia, was significantly less than the volume in matched controls25. Other chronic conditions, such as, diabetes, stroke, and cancer, have also been associated with late-onset hypogonadism in men, and thus, it appears that a diminished testosterone level is common response to chronic disease26. However, our finding of a linear association between testicular volume and pulmonary function may suggest a potential link between gonadal and pulmonary functions.
The literature is somewhat inconsistent regarding the relationship between smoking and testosterone levels. Most cross-sectional studies indicate that smoking increases total testosterone and that smoking cessation reduces testosterone levels in men. As SHBG levels are elevated in smoking men, it has been suggested that this reported increase in testosterone levels is a consequence of increased SHBG levels2728. However, Kirbas et al.28 studied the association between hypogonadism, smoking, and obstructive sleep apnea and found no relationship between current smoking and testosterone levels. In the present study, univariate analysis using linear regression models showed that both testicular volume and testosterone levels were not correlated with smoking.
The human testis has been evaluated in term of its endocrine function, daily sperm output in ejaculates, general appearance of seminiferous tubules, differential cell counts in the testis, and daily sperm production29. Although many reports regarding Prader's orchiometer-derived testicular volume are available, no uniform reference values are available, due to differences in the nature of the populations studied (geographic area, nutritional status, ethnicity, and environmental factors)111330. The normal range of one testicular volume has been reported to be 14–30 mL12133132, but human testicular volumes are markedly dependent on ethnicity 303334. For example, testicular volumes have been reported to greater in Caucasian than in Oriental populations34. As compared with the results of Auger and Eustache35, our study population had smaller total testicular volumes (34.7±9.2 mL vs. 55.7±18.7 mL) (Table 1), and thus, it is not appropriate to compare our Korean data with Caucasian data directly. However, testicular volumes in this study were similar to those found in largescale studies of healthy young Korean men1117, which means testicular volumes in our study population were within normal limits, as we excluded patients with conditions known to influence testicular volume.
Like lung function18, clinically assessed testicular volume varies with age36. In a large cohort of males aged 0–28 years, maximum testicular volume was attained at 17–18 and 21–22 years among non-gypsies and gypsies, respectively37. Findings of testicular volume declines with aging are not universal, as some have reported a decrease1920 and others no change38. It has also been reported age, malnutrition and illness exert independent effects on testicular volume39, and that age is associated with a reduction in testicular volume only in the eighth decade of life3940. However, recent studies suggest that mild testicular volume decline often occurs earlier from the 50 or 60s4142. Reduced testicular volume is associated with hormonal abnormalities, including low testosterone and increased luteinizing hormone (LH) and follicle-stimulating hormone (FSH) levels1242. In aged men, testicular volume, testicular parenchymal weight, and daily sperm production are significantly reduced29. In addition, plasma LH, FSH, and estradiol concentrations are elevated whereas plasma testosterone, free testosterone, and the ability of the testis to secrete testosterone following stimulation are diminished. However, testosterone levels can fluctuate substantially day-to-day and sometimes within days. Therefore, testicular volume, which is relatively stable, represents androgen status better than a single measurement of testosterone level.
Several studies have reported a positive relationship exists between testicular volume and body weight1117. In the present study, height, weight, and body mass index exhibited significant association with testicular volumes by univariate analysis (Table 2). However, body weight was not found to independently predict FEV1 % predicted by multivariate analysis. In fact, only smoking and testicular volume were found to independently predict FEV1 % predicted (Table 3).
We enrolled the relatively small number of subjects from a specific setting. Furthermore, our results cannot be applied to other populations, given the limited demographics of the study population. Another limitation is that we used prebronchodilator spirometric values to evaluate lung function, and thus, we cannot rule out the possibility of transient airflow obstruction in some patients.
Summarizing, although the present study was not conducted using a large-scale, longitudinal design, it shows larger testicular volume was independently associated with favorable indices of lung function, and suggests that androgens may contribute to better lung function.
References
1. Laghi F, Langbein WE, Antonescu-Turcu A, Jubran A, Bammert C, Tobin MJ. Respiratory and skeletal muscles in hypogonadal men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005; 171:598–605. PMID: 15591465.

2. Kamischke A, Kemper DE, Castel MA, Luthke M, Rolf C, Behre HM, et al. Testosterone levels in men with chronic obstructive pulmonary disease with or without glucocorticoid therapy. Eur Respir J. 1998; 11:41–45. PMID: 9543268.

3. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR;. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001; 86:724–731. PMID: 11158037.
4. Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002; 87:589–598. PMID: 11836290.

5. Daabis RG, Rehem RN, Hassan MM, Khalil GI. Hypogonadism in patients with chronic obstructive pulmonary disease: relationship with airflow limitation, muscle weakness and systemic inflammation. Alexandria J Med. 2016; 52:27–33.

6. Van Vliet M, Spruit MA, Verleden G, Kasran A, Van Herck E, Pitta F, et al. Hypogonadism, quadriceps weakness, and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005; 172:1105–1111. PMID: 16100014.

7. Laghi F, Antonescu-Turcu A, Collins E, Segal J, Tobin DE, Jubran A, et al. Hypogonadism in men with chronic obstructive pulmonary disease: prevalence and quality of life. Am J Respir Crit Care Med. 2005; 171:728–733. PMID: 15657463.
8. Debigare R, Marquis K, Cote CH, Tremblay RR, Michaud A, LeBlanc P, et al. Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest. 2003; 124:83–89. PMID: 12853506.

9. Svartberg J, Schirmer H, Medbo A, Melbye H, Aasebo U. Reduced pulmonary function is associated with lower levels of endogenous total and free testosterone. The Tromso study. Eur J Epidemiol. 2007; 22:107–112. PMID: 17260104.
10. Wheeler MJ, Barnes SC. Measurement of testosterone in the diagnosis of hypogonadism in the ageing male. Clin Endocrinol (Oxf). 2008; 69:515–525. PMID: 18616710.

11. Bahk JY, Jung JH, Jin LM, Min SK. Cut-off value of testes volume in young adults and correlation among testes volume, body mass index, hormonal level, and seminal profiles. Urology. 2010; 75:1318–1323. PMID: 20299083.

12. Sakamoto H, Ogawa Y, Yoshida H. Relationship between testicular volume and testicular function: comparison of the Prader orchidometric and ultrasonographic measurements in patients with infertility. Asian J Androl. 2008; 10:319–324. PMID: 18097521.

13. Takihara H, Cosentino MJ, Sakatoku J, Cockett AT. Significance of testicular size measurement in andrology: II. Correlation of testicular size with testicular function. J Urol. 1987; 137:416–419. PMID: 3102757.

14. Preston BT, Stevenson IR, Lincoln GA, Monfort SL, Pilkington JG, Wilson K. Testes size, testosterone production and reproductive behaviour in a natural mammalian mating system. J Anim Ecol. 2012; 81:296–305. PMID: 21958300.

15. Rey RA, Campo SM, Bedecarras P, Nagle CA, Chemes HE. Is infancy a quiescent period of testicular development? Histological, morphometric, and functional study of the seminiferous tubules of the cebus monkey from birth to the end of puberty. J Clin Endocrinol Metab. 1993; 76:1325–1331. PMID: 8496325.

16. Ankarberg-Lindgren C, Norjavaara E. Changes of diurnal rhythm and levels of total and free testosterone secretion from pre to late puberty in boys: testis size of 3 ml is a transition stage to puberty. Eur J Endocrinol. 2004; 151:747–757. PMID: 15588242.

17. Ku JH, Kim ME, Jeon YS, Lee NK, Park YH. Factors influencing testicular volume in young men: results of a communitybased survey. BJU Int. 2002; 90:446–450. PMID: 12175406.

18. Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977; 1:1645–1648. PMID: 871704.

19. Stearns EL, MacDonnell JA, Kaufman BJ, Padua R, Lucman TS, Winter JS, et al. Declining testicular function with age: hormonal and clinical correlates. Am J Med. 1974; 57:761–766. PMID: 4140691.
20. Baker HW, Burger HG, de Kretser DM, Hudson B, O'Connor S, Wang C, et al. Changes in the pituitary-testicular system with age. Clin Endocrinol (Oxf). 1976; 5:349–372. PMID: 971543.

21. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338. PMID: 16055882.
22. Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187:347–365. PMID: 22878278.
23. Kalyani RR, Gavini S, Dobs AS. Male hypogonadism in systemic disease. Endocrinol Metab Clin North Am. 2007; 36:333–348. PMID: 17543722.

24. Balasubramanian V, Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr Opin Pulm Med. 2012; 18:112–117. PMID: 22234275.
25. Gosney JR. Atrophy of Leydig cells in the testes of men with longstanding chronic bronchitis and emphysema. Thorax. 1987; 42:615–619. PMID: 3660314.

26. Isidori AM, Lenzi A. Risk factors for androgen decline in older males: lifestyle, chronic diseases and drugs. J Endocrinol Invest. 2005; 28(3 Suppl):14–22.
27. English KM, Pugh PJ, Parry H, Scutt NE, Channer KS, Jones TH. Effect of cigarette smoking on levels of bioavailable testosterone in healthy men. Clin Sci (Lond). 2001; 100:661–665. PMID: 11352783.

28. Kirbas G, Abakay A, Topcu F, Kaplan A, Unlu M, Peker Y. Obstructive sleep apnoea, cigarette smoking and serum testosterone levels in a male sleep clinic cohort. J Int Med Res. 2007; 35:38–45. PMID: 17408053.

29. Johnson L. Evaluation of the human testis and its age-related dysfunction. Prog Clin Biol Res. 1989; 302:35–60. PMID: 2666991.
30. Diamond JM. Ethnic differences: variation in human testis size. Nature. 1986; 320:488–489. PMID: 3083267.

31. Lenz S, Giwercman A, Elsborg A, Cohr KH, Jelnes JE, Carlsen E, et al. Ultrasonic testicular texture and size in 444 men from the general population: correlation to semen quality. Eur Urol. 1993; 24:231–238. PMID: 8104150.

32. Arai T, Kitahara S, Horiuchi S, Sumi S, Yoshida K. Relationship of testicular volume to semen profiles and serum hormone concentrations in infertile Japanese males. Int J Fertil Womens Med. 1998; 43:40–47. PMID: 9532468.
33. Dave DS, Tiwari VD. Normal testicular volume in school children of low socio-economic group. Indian Pediatr. 1983; 20:901–905. PMID: 6609884.
34. Mittwoch U. Ethnic differences in testis size: a possible link with the cytogenetics of true hermaphroditism. Hum Reprod. 1988; 3:445–449. PMID: 3392179.

35. Auger J, Eustache F. Second to fourth digit ratios, male genital development and reproductive health: a clinical study among fertile men and testis cancer patients. Int J Androl. 2011; 34(4 Pt 2):e49–e58. PMID: 21091719.

36. Lotti F, Maggi M. Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update. 2015; 21:56–83. PMID: 25038770.

37. Beres J, Papp G, Pazonyi I, Czeizel E. Testicular volume variations from 0 to 28 years of age. Int Urol Nephrol. 1989; 21:159–167. PMID: 2744988.
38. Harman SM, Tsitouras PD. Reproductive hormones in aging men. I. Measurement of sex steroids, basal luteinizing hormone, and Leydig cell response to human chorionic gonadotropin. J Clin Endocrinol Metab. 1980; 51:35–40. PMID: 7189758.

39. Handelsman DJ, Staraj S. Testicular size: the effects of aging, malnutrition, and illness. J Androl. 1985; 6:144–151. PMID: 3997660.

40. Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update. 2010; 16:65–79. PMID: 19696093.

41. Pilatz A, Rusz A, Wagenlehner F, Weidner W, Altinkilic B. Reference values for testicular volume, epididymal head size and peak systolic velocity of the testicular artery in adult males measured by ultrasonography. Ultraschall Med. 2013; 34:349–354. PMID: 23165790.

42. Rastrelli G, Corona G, Lotti F, Boddi V, Mannucci E, Maggi M. Relationship of testis size and LH levels with incidence of major adverse cardiovascular events in older men with sexual dysfunction. J Sex Med. 2013; 10:2761–2773. PMID: 23844651.

Table 1
Characteristics of the study population
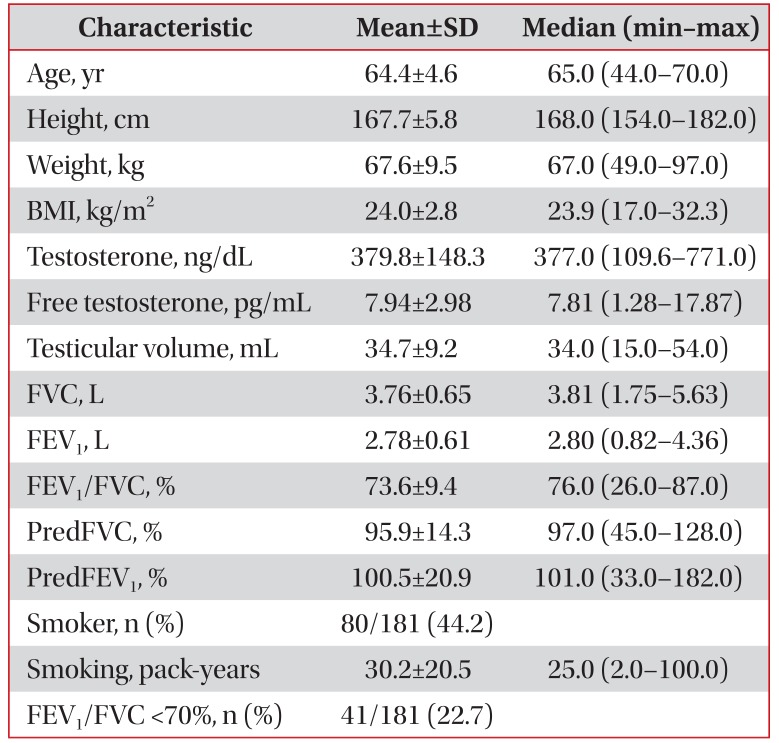
Table 2
Relationships between PFT findings and study variables (univariate analysis using linear regression models) (n=181)
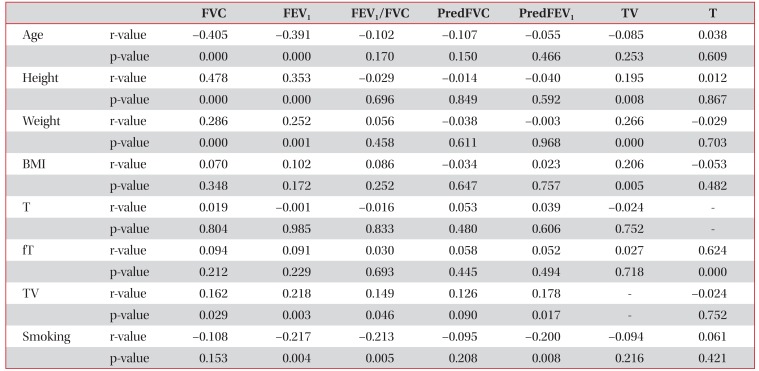
Table 3
Multivariate analysis using linear regression models (n=181)
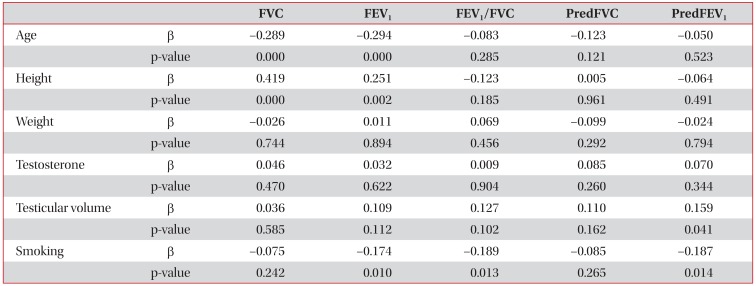
Table 4
Characteristics of patients in two study groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download