This article has been corrected. See "Corrigendum: Elucidation of Bacterial Pneumonia-Causing Pathogens in Patients with Respiratory Viral Infection" in Volume 81 on page 349.
Abstract
Background
Bacterial pneumonia occurring after respiratory viral infection is common. However, the predominant bacterial species causing pneumonia secondary to respiratory viral infections other than influenza remain unknown. The purpose of this study was to know whether the pathogens causing post-viral bacterial pneumonia vary according to the type of respiratory virus.
Methods
Study subjects were 5,298 patients, who underwent multiplex real-time polymerase chain reaction for simultaneous detection of respiratory viruses, among who visited the emergency department or outpatient clinic with respiratory symptoms at Ulsan University Hospital between April 2013 and March 2016. The patients' medical records were retrospectively reviewed.
Results
A total of 251 clinically significant bacteria were identified in 233 patients with post-viral bacterial pneumonia. Mycoplasma pneumoniae was the most frequent bacterium in patients aged <16 years, regardless of the preceding virus type (p=0.630). In patients aged ≥16 years, the isolated bacteria varied according to the preceding virus type. The major results were as follows (p<0.001): pneumonia in patients with influenza virus (type A/B), rhinovirus, and human metapneumovirus infections was caused by similar bacteria, and the findings indicated that Staphylococcus aureus pneumonia was very common in these patients. In contrast, coronavirus, parainfluenza virus, and respiratory syncytial virus infections were associated with pneumonia caused by gram-negative bacteria.
Respiratory viral infection is a significant etiology for community-acquired pneumonia123. With the development of detection techniques, respiratory viruses have been detected in 10%–30% patients with community-acquired pneumonia45. Respiratory virus infections are also a frequent cause of bacterial pneumonia. In a systematic review of previous studies, the proportion of bacterial pneumonia in patients with influenza was found to range between 11% and 35%6.
In a study of pandemic and seasonal influenza virus infections, the most common bacterial pathogens found in patients with post-influenza pneumonia were Staphylococcus aureus and Streptococcus pneumoniae78. Elucidation of the pneumonia-causing pathogens in patients with respiratory viral infection is important, because respiratory viral infection complicating bacterial pneumonia is associated with a worse prognosis and high mortality rate compared with respiratory viral infection only 9, although the prognosis can be improved with early and appropriate empirical antibiotic treatment7910. However, the predominant bacterial species causing pneumonia secondary to respiratory viral infections other than influenza remain unknown. Accordingly, the aim of the present study was to know whether the pathogens causing post-viral bacterial pneumonia vary according to the type of preceding respiratory virus.
The primary study subjects were 5,298 patients, who underwent multiplex real-time polymerase chain reaction (PCR) for simultaneous detection of respiratory viruses, among who visited the emergency department or outpatient clinic with respiratory symptoms at Ulsan University Hospital from April 2013 to March 2016 (Figure 1). The medical records of all these patients were retrospectively reviewed in detail.
We used a commercial multiplex real-time PCR kit (LG Life Sciences, Seoul, Korea) that can simultaneously detect nine respiratory viruses (adenovirus, bocavirus, coronavirus, type A influenza virus, type B influenza virus, human metapneumovirus [hMPV], parainfluenza virus, human rhinovirus, and respiratory syncytial virus [RSV]) in respiratory specimens.
Using chest radiography and/or computed tomography (CT) performed within 48 hours of the hospital visit, patients meeting one or more of the following criteria were assigned a diagnosis of pneumonia: new or progressive infiltrates (lobar, lobular, nodular, or diffuse), increased interstitial lung marking (unilateral>bilateral), and pleural effusion and loculated fluid collection on radiographs and/or CT images1112. Postviral pneumonia was defined as the presence of pneumonia (as described above) in a patient with respiratory viral infection. Post-viral bacterial pneumonia was defined as the above (post-viral pneumonia) plus simultaneous detection of pathogenic bacteria in a respiratory specimen.
To know bacterial pneumonia-causing pathogens in patients with respiratory viral infection, the results of the following tests performed within 48 hours of the hospital visit were investigated for patients with virus positivity and (radiological) pneumonia (i.e., post-viral pneumonia): culture for gram-positive or gram-negative bacteria (blood, sputum, endotracheal aspirate, bronchoalveolar lavage or washing, or pleural fluid), serum enzyme immunoassay (EIA) for the detection of IgM of Chlamydia pneumoniae and Mycoplasma pneumoniae, PCR of respiratory secretions for the detection of C. pneumoniae and M. pneumoniae, and detection of urinary antigens for Legionella pneumophila and S. pneumoniae2. Coagulase-negative staphylococci and viridans streptococci were excluded from the analysis because they were considered insignificant.
The primary objective of the present study was to determine whether pathogens causing post-viral bacterial pneumonia varied according to the type of respiratory virus. Therefore, we investigated the distribution of bacteria by the type of preceding virus in patients with radiological pneumonia. In addition, the incidence of post-viral pneumonia according to the type of respiratory virus and the seasonal and age-specific distribution of respiratory viral infection (to compare data with previous studies) were surveyed.
Categorical variables are expressed as numbers and percentages. Fisher exact tests and the chi-square tests were used to evaluate statistical significance. Continuous variables are expressed as means and standard deviations (SD). Independent t test was used to evaluate statistical significance. All statistical analyses were performed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
Over the 3-year study period (April 2013 to March 2016), respiratory viruses were detected in 3,328 of the 5,298 patients (62.8%) by multiplex real-time PCR. Of the 3,328 patients, 704 (21.2%) were diagnosed with post-viral pneumonia according to chest radiography and/or chest CT findings (in these patients, nasopharyngeal swab [653/704, 92.8%] and bronchoalveolar lavage/bronchial washing fluid [51/704, 7.2%] were used to detect respiratory virus). A total of 251 clinically significant bacterial pathogens were identified in 233 of the 704 patients (33.1%) with pneumonia (Figure 1).
The most common virus identified in the present study was human rhinovirus (29.7%), followed by RSV (26.4%), parainfluenza virus (9.6%), adenovirus (9.4%), hMPV (6.3%), coronavirus (6.1%), type A influenza virus (5.6%), bocavirus (4.5%), and type B influenza virus (2.4%) (p<0.001) (Table 1, Figure 2).
Regardless of the type of virus, respiratory viral infection showed two age peaks, i.e., <10 years and approximately 60 years. Infections caused by coronavirus, type A influenza virus, hMPV, human rhinovirus, and RSV occurred not only in infants and children but also in adults aged 60 to 80 years. However, type B influenza and adenovirus infections showed a different age distribution: they occurred mainly in adolescents, middle-aged adults (40–60 years old), and infants and younger children (Figure 3).
Each viral infection exhibited a specific seasonal distribution, as shown in Figure 4. Infections caused by adenovirus, coronavirus, type A influenza virus, and RSV occurred mainly in winter; those caused by bocavirus and hMPV occurred most commonly in spring, and those caused by type B influenza virus occurred most commonly in late winter and early spring (a little later than type A influenza). Parainfluenza infection occurred most commonly in spring and summer, although it was roughly perennial. Rhinovirus infection exhibited a perennial pattern.
Among the 704 patients with post-viral pneumonia, the most frequent pneumonia-causing virus was RSV (26.0%), followed by human rhinovirus (24.4%), hMPV (10.9%), parainfluenza virus (8.7%), type A influenza virus (8.4%), adenovirus (7.1%), coronavirus (6.3%), bocavirus (5.5%), and type B influenza virus (2.8%). Patients with hMPV infections exhibited the highest incidence of post-viral pneumonia (76/209, 36.4%), followed by patients with type A influenza virus (59/188, 31.4%), bocavirus (39/151, 25.8%), and type B influenza virus (20/80, 25.0%) infections (p<0.001). The rate of bacterial identification (i.e., post-viral bacterial pneumonia) was the highest for patients with type B influenza virus infection (9/20, 45.0%), followed by those with coronavirus (19/44, 43.2%), type A influenza virus (25/59, 42.4%), human rhinovirus (73/172, 42.4%), adenovirus (20/50, 40.0%), and hMPV (24/77, 31.2%) infections (Table 1, Figure 5). Comparing to the patients with post-viral pneumonia, those with post-viral bacterial pneumonia received significantly more intensive and ventilator care, and had a higher mortality rate (Table 2).
With reference to previous studies, we divided the patients with post-viral bacterial pneumonia (n=233) into those aged <16 years (130/233 [55.8%]; mean±SD, 4.66±2.84 years) and those aged ≥16 years (103/233 [44.2%]; mean±SD, 64.91±15.95 years)1314. In the <16 year age group, there were 61 boys (47.0%) and 69 girls (53.0%), while in the ≥16 year age group, there were 65 men (63.0%) and 38 women (37.0%). Four patients (3.0%) in the <16 year age group and 43 (41.0%) in the ≥16 year age group required care in the intensive care unit. Three patients (2.0%) in the <16 year age group and 26 (25.0%) in the ≥16 year age group required mechanical ventilator care. During the study period, none of the patients in the <16 year age group died; however, there were 21 deaths (20.3%) in the ≥16 year age group. The baseline characteristics and comorbidities for our study population are presented in Table 3.
In the <16 year age group, M. pneumoniae was the most frequently identified bacterium, regardless of the preceding virus type (p=0.630). In the ≥16 year age group, the isolated bacteria varied according to the preceding virus type (p<0.001). Type A influenza preceded bacterial infections with S. aureus (6/21), Klebsiella spp. (4/21), S. pneumoniae (3/21), and Acinetobacter spp. (3/21); type B influenza preceded bacterial infections with S. aureus (2/9), S. pneumoniae (2/9), and Acinetobacter spp. (2/9); hMPV infection preceded bacterial infections with S. aureus (4/21), Klebsiella spp. (4/21), and Acinetobacter spp. (4/21); human rhinovirus infection preceded bacterial infections with S. aureus (7/24), Klebsiella spp. (3/24), C. pneumoniae (3/24), and Pseudomonas spp. (3/24); coronavirus infection preceded bacterial infections with Acinetobacter spp. (3/13), Klebsiella spp. (3/13), and Pseudomonas spp. (2/13); parainfluenza virus infection preceded bacterial infections with Acinetobacter spp. (2/10), Klebsiella spp. (2/10), and M. pneumoniae (2/10); and RSV infection preceded bacterial infections with Escherichia coli (3/13), Acinetobacter spp. (3/13), and Enterococcus spp. (2/13) (Table 4). Distribution of identified bacteria by specimen type was showed at Table 5.
As bacterial pneumonia in patients with respiratory viral infection is associated with a poor prognosis and increased mortality, we tried to know whether bacterial pneumonia-causing pathogens varied according to the type of the preceding respiratory virus. On the basis of our results, we found that the cause of bacterial pneumonia in adults with respiratory viral infection varies with the preceding virus type. Specifically, influenza type A/B virus, rhinovirus, and hMPV infections primarily led to pneumonia caused by S. aureus, while coronavirus, parainfluenza virus, and RSV infections primarily led to pneumonia caused by gram-negative rods. In children, M. pneumonia was the most frequent pneumonia-causing bacterium, regardless of the type of preceding virus. Additionally, we found that the incidences of post-viral pneumonia and post-viral bacterial pneumonia varied according to the virus type; hMPV was associated with the highest incidence of post-viral pneumonia, while type B influenza virus was associated with the highest rate of post-viral bacterial pneumonia.
A previous study reported that there were no significant differences in symptoms, demographic characteristics, and hospital visit frequency between respiratory viral infection patients with and without post-viral bacterial pneumonia8. Nevertheless, it is important to distinguish between the two groups, because patients with respiratory viral infection and post-viral bacterial pneumonia exhibit a worse prognosis compared with patients with respiratory viral infection only9. The prognosis of the former patients could be improved by the selection of appropriate empirical antibiotics according to the bacterial pathogen that is significantly associated with the culprit respiratory virus type7.
In patients aged ≥16 years in the present study, type A/B influenza virus infections preceded bacterial pneumonia caused by S. aureus, Klebsiella spp., S. pneumoniae, and Acinetobacter spp. Unlike in previous studies, Haemophilus influenzae and S. pyogenes were not identified567. Instead, gramnegative bacteria such as Klebsiella spp. and Acinetobacter spp. were identified. Bacterial pathogens in patients with human rhinovirus infection included S. aureus, Pseudomonas spp., Klebsiella spp., and C. pneumoniae. In another study, post-rhinovirus bacterial pathogens included H. influenzae, S. pneumoniae, and M. catarrhalis15. hMPV infection preceded pneumonia caused by S. aureus, Klebsiella spp., and Acinetobacter spp. In a previous study, six patients with community-acquired pneumonia had hMPV infection and complicating bacterial pneumonia with S. pneumoniae, M. pneumoniae, or C. pneumoniae16. The other virus-bacteria associations (in our study) were as follows: coronavirus—Acinetobacter spp., Klebsiella spp., and Pseudomonas spp.; parainfluenza virus—Acinetobacter spp., Klebsiella spp., and M. pneumoniae; and RSV—E. coli, Acinetobacter spp., and Enterococcus spp. In summary, pneumonia in patients with influenza virus (type A/B), rhinovirus, and hMPV infections was caused by similar bacteria, and the findings indicated that S. aureus pneumonia was very common in these patients. In contrast, coronavirus, parainfluenza virus, and RSV infections were associated with pneumonia caused by gram-negative bacteria.
In the <16 year age group in the present study, the most common pathogen causing bacterial pneumonia secondary to viral infection was M. pneumoniae. In a previous study determining pathogens in children, S. pneumoniae, M. pneumoniae, and C. pneumoniae were the commonly detected bacteria causing post-viral bacterial pneumonia13. Despite the retrospective study design and the difficulty of sampling in children, the detection rate of M. pneumonia was very high in the present study.
In our study, hMPV was found to be the most common virus complicating pneumonia, followed by type A influenza. This is an interesting finding that has been reported in previous studies as well. One study compared viruses in 450 asymptomatic adults and 183 adult patients with pneumonia17 and reported significantly higher detection rates for influenza virus, RSV, and hMPV in patients with pneumonia. In another study, RSV and hMPV in children, and rhinovirus and hMPV in adults, were strongly associated with community-acquired pneumonia18. Yet another study suggested that hMPV frequently induces pneumonia complicating acute respiratory distress syndrome (ARDS) in patients without significant comorbidities or immunosuppression19. Taken together, hMPV can cause more serious diseases, such as pneumonia and ARDS, compared with influenza virus. Therefore, research on hMPV, including the development of therapeutic agents, is urgently required. In addition, we found that secondary bacterial pneumonia were relatively common in patients with pneumonia secondary to influenza, coronavirus, rhinovirus, adenovirus, and hMPV infections, a finding that has not been reported previously. Our results suggest that in patients with pneumonia and confirmed infection with the above mentioned viruses, it is necessary to pay more attention to the occurrence of bacterial pneumonia.
This study has some limitations because of its retrospective design. First, for patients aged <16 years, sufficient sputum culture tests were not performed in comparison with blood culture and serological tests. Therefore, except mycoplasma and chlamydia, which can be determined by serology and PCR, other bacterial pathogens are likely to have been underestimated. Second, since we used single titer IgM detection EIA, the prevalence of C. pneumoniae and M. pneumoniae could be overestimated. Third, we did not evaluate the underlying disease (malnutrition, steroid use) and lung condition (bronchiectasis, chronic obstructive pulmonary disease) that could affect bacterial colonization. To consolidate our findings, additional prospective studies are necessary. Forth, the study sample was small, which may affect the generalizability of our results.
In conclusion, the present study demonstrated that postviral bacterial pneumonia-causing pathogens differ according to the type of the culprit respiratory virus. Despite the study limitations, the findings are significant because of the lack of previous research on the etiology of secondary bacterial pneumonia in patients with respiratory virus infections other than influenza, and they will aid in the appropriate selection of empirical antibiotics for patients with (proven) respiratory viral infection and (radiological) pneumonia. For the treatment of post-viral pneumonia in infants and children, antibiotics with activity against M. pneumoniae (e.g., macrolides) should be considered. In adults, antistaphylococcal antibiotics should be considered when pneumonia occurs in patients with influenza virus (type A/B), rhinovirus, and hMPV infections, while antibiotics against a wide range of gram-negative bacteria should be considered when pneumonia occurs after coronavirus and parainfluenza virus infections. To confirm our retrospective results, further replicative prospective studies are needed.
Figures and Tables
 | Figure 2Incidence of respiratory viral infection in the study population between April 2013 and March 2016 (p<0.001). hMPV: human metapneumovirus; RSV: respiratory syncytial virus. |
 | Figure 4Seasonal distribution of respiratory viruses between April 2013 and March 2016 (spring: March–May, summer: June–August, fall: September–November, winter: December–February). |
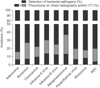 | Figure 5Incidence of pneumonia (p<0.001) and bacterial pneumonia (p<0.001) associated with respiratory viral infection. RSV: respiratory syncytial virus; CT: computed tomography. |
Table 1
Incidence of post-viral pneumonia and post-viral bacterial pneumonia

Table 2
Outcome comparison of post-viral pneumonia and post-viral bacterial pneumonia

Table 3
Baseline characteristics of 233 post-viral bacterial pneumonia patients

Values are presented as mean±SD or number (%).
*Any underlying medical condition including chronic lung disease (asthma, chronic obstructive pulmonary disease, interstitial lung disease, and bronchiectasis), chronic heart disease (coronary artery disease or congestive heart failure, hypertension, and valvulopathy), diabetes mellitus, chronic kidney disease (with or without dialysis), neurologic disorders (epilepsy, cerebral palsy, dementia, or history of stroke), and chronic liver disease (hepatitis, cirrhosis, or hepatic failure). The groups were not mutually exclusive.
Table 4
Identified bacterial pathogens in patients with post-viral bacterial pneumonia

Table 5
Distribution of identified bacteria by specimen type

Acknowledgments
This work was funded by Ulsan University Hospital (Biomedical Research Center Promotion Fund, UUH-06-15).
References
1. Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015; 373:415–427.
2. Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015; 372:835–845.
3. Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007; 44:Suppl 2. S27–S72.
4. American Thoracic Society. Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005; 171:388–416.
5. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012; 18:268–281.
6. Klein EY, Monteforte B, Gupta A, Jiang W, May L, Hsieh YH, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016; 10:394–403.
7. Chertow DS, Memoli MJ. Bacterial coinfection in influenza: a grand rounds review. JAMA. 2013; 309:275–282.
8. Rice TW, Rubinson L, Uyeki TM, Vaughn FL, John BB, Miller RR 3rd, et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med. 2012; 40:1487–1498.
9. Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006; 34:1589–1596.
10. Rello J, Gallego M, Mariscal D, Sonora R, Valles J. The value of routine microbial investigation in ventilator-associated pneumonia. Am J Respir Crit Care Med. 1997; 156:196–200.
11. Watt JP, Moisi JC, Donaldson RL, Reid R, Ferro S, Whitney CG, et al. Measuring the incidence of adult community-acquired pneumonia in a Native American community. Epidemiol Infect. 2010; 138:1146–1154.
12. Cherian T, Mulholland EK, Carlin JB, Ostensen H, Amin R, de Campo M, et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005; 83:353–359.
13. Lehtinen P, Jartti T, Virkki R, Vuorinen T, Leinonen M, Peltola V, et al. Bacterial coinfections in children with viral wheezing. Eur J Clin Microbiol Infect Dis. 2006; 25:463–469.
14. Khadadah M, Essa S, Higazi Z, Behbehani N, Al-Nakib W. Respiratory syncytial virus and human rhinoviruses are the major causes of severe lower respiratory tract infections in Kuwait. J Med Virol. 2010; 82:1462–1467.
15. Pitkaranta A, Roivainen M, Blomgren K, Peltola J, Kaijalainen T, Raty R, et al. Presence of viral and bacterial pathogens in the nasopharynx of otitis-prone children: a prospective study. Int J Pediatr Otorhinolaryngol. 2006; 70:647–654.
16. Lin PY, Lin TY, Huang YC, Tsao KC, Huang YL. Human metapneumovirus and community-acquired pneumonia in children. Chang Gung Med J. 2005; 28:683–688.
17. Lieberman D, Shimoni A, Shemer-Avni Y, Keren-Naos A, Shtainberg R, Lieberman D. Respiratory viruses in adults with community-acquired pneumonia. Chest. 2010; 138:811–816.
18. Self WH, Williams DJ, Zhu Y, Ampofo K, Pavia AT, Chappell JD, et al. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis. 2016; 213:584–591.
19. Hasvold J, Sjoding M, Pohl K, Cooke C, Hyzy RC. The role of human metapneumovirus in the critically ill adult patient. J Crit Care. 2016; 31:233–237.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download