Abstract
Ullrich congenital muscular dystrophy (UCMD) is caused by mutations in one of three genes encoding collagen VI. Although UCMD usually shows an early onset, progressive weakness, contractures and hyperlaxity of the joints, and respiratory failure, it is well known to exhibit a wide spectrum of clinical severities. The severities of the phenotypic subtypes are mainly divided according to the ambulation status. We report a patient with the early-severe phenotype of UCMD who was diagnosed by the detection of novel recessive mutations in COL6A1.
Ullrich congenital muscular dystrophy (UCMD) is caused by either dominant or recessive mutations in one of three genes encoding collagen VI (COL6A1, COL6A2, and COL6A3), which is a major component of the extracellular matrix in skeletal muscles.1 UCMD is therefore categorized into collagen-VI-related myopathies that also include Bethlem myopathy (BM) and an intermediate myopathy between UCMD and BM.2 UCMD is clinically characterized by the early onset of muscle weakness, proximal joint contractures, hyperlaxity of distal joints, and progressive respiratory failure. However, a significant diversity is present in the phenotypic spectrum of UCMD, and three subtypes are defined according to severity: early-severe, moderate-progressive, and mild phenotypes.34 We report a patient with the early-severe phenotype of UCMD, in whom the diagnosis was confirmed by the detection of novel recessive mutations in COL6A1.
A 4-year-old boy presented with developmental delay. He had been delivered as a low-birth-weight infant at a gestational age of 34 weeks, and no perinatal complication was reported. He began to hold up his head at 6 months of age, but had achieved no further motor milestones by the age of 2 years. An examination revealed that he could crawl but would never sit alone, stand, or walk, even with assistance. His muscle power was graded as 2 or 3 in the upper and lower extremities, and the muscle tone was generally decreased. Facial weakness and a high-arched palate were present. Knee contractures were predominant bilaterally (Fig. 1A), and hip adductor contractures had been operated on at the age of 2 years. Distal joints such as of the wrist, fingers, and toes were markedly hyperextensible (Fig. 1B). Characteristic calcaneal protuberance was noted (Fig. 1C). The serum creatine kinase level was 198-243 IU/L. Electrophysiological studies revealed positive sharp waves, fibrillation potentials, and small polyphasic motor-unit potentials in the right vastus lateralis and biceps muscles. He did not complain of respiratory difficulty at any time up to the last follow-up.
Muscle pathology showed mostly round-shaped muscle fibers with marked size variations (Fig. 2A). Endomysial connective tissue was remarkably increased in the areas sampled, but necrotic and regenerating fibers were not present. Immunohistochemical staining showed collagen VI was moderately reduced relative to a disease control (Fig. 2B, C), but localized to the sarcolemma and extracellular matrix. Considering that the characteristic clinical and pathological features were suggestive of UCMD, Sanger sequencing was performed for COL6A1, COL6A2, and COL6A3. Two novel mutations were found in CO6LA1, one of which was a nucleotide change in the first base of the start codon (c.1A > G). If the next start codon located at position 62 is working, a new reading frame terminates prematurely at 30 codons downstream. The second mutation was a single-nucleotide deletion (c.504delC), which was also expected to cause premature truncation (p.Cys169Valfs*7). These mutations have not been reported before, and they were also not found in 200 normal control chromosomes. To evaluate whether protein loss was induced by transcription failure, the transcript of COL6A1 was quantified by the quantitative reverse-transcription polymerase chain reaction (RT-PCR). The amount of the COL6A1 transcript was markedly lower for the patient compared to a normal control (28.6% of the normal value; Fig. 2D).
This case was highly representative of the early-severe phenotype of UCMD. The ambulation status is considered a key factor for dividing the phenotypic subtypes according to disease severity.3 Because this patient could only crawl by himself and had never achieved independent ambulation, he was categorized into the early-severe phenotype. In contrast, a loss of ambulation after its initial achievement is categorized as the moderate-progressive phenotype, and the maintenance of ambulation until adulthood is categorized as the mild phenotype.34 We did not evaluate his respiratory function due to follow-up loss, at which time he had not yet complained of respiratory difficulty, but most patients with the early-severe phenotype are known to experience early respiratory failure at around 10 years old.45 This most-severe presentation of UCMD reportedly accounts for 18.0–25.7% of cases.4 However, it seems to be very rare in Korean cohorts, with a study that involved 22 cases of collagen-VI-related myopathies including no case of the early-severe phenotype.6 Full manifestations of joint abnormalities including proximal contractures and distal hyperlaxity, and also calcaneal protuberance were remarkable in the present case. According to one report, the early-severe phenotype tends to show a higher incidence of joint abnormalities such as contractures and spinal deformities.3
Based on clinical findings, we initially focused on the collagen VI genes, and finally identified novel recessive mutations in COL6A1. One of the novel mutations was a deletion of a single nucleotide, which is thought to cause a premature termination codon (PTC). In addition, it was located before the triple helical (TH) domain that is known to be critical for collagen assembly.7 This might have had a deleterious effect on the production of collagen VI. It was more remarkable that the other mutation occurred on the first base of the start codon. The presence of a start-codon mutation would usually result in translation failing to produce a null allele. Translation can occur if an alternate start codon is present adjacent to the original one, but this would be incomplete and so cause premature truncation.8 Thus, both mutations were expected to result in a significant reduction in the protein level. This was supported by our finding that the amount of protein revealed by immunohistochemical staining and the transcript level revealed by quantitative RT-PCR were both markedly decreased in the patient compared with a disease control and a normal control. In our patient the measured transcript was mostly from the allele carrying the c.504delC deletion mutation, since transcription in the other allele would be prohibited. There is a previous report of all the cases of the early-severe phenotype having recessive PTC-causing mutations, especially involving the TH domain.3 Also, the absence of the early-severe phenotype in a previously reported Korean cohort was possibly due to none of them carrying PTC-causing mutations.6 However, this relationship is not always present since a few of the patients with milder manifestations are found to have recessive PTC-causing mutations.34 The phenotype-genotype correlation seems to be complicated in UCMD, and it has been hypothesized that polymorphisms in COL6A contribute to the variable clinical severity.3
In conclusion, this case clearly shows that deleterious effects caused by a start-codon mutation and a PTC-causing mutation can result in the early-severe phenotype of UCMD. Although this is very rare, it may guide genetic searches when this kind of presentation is encountered.
Figures and Tables
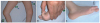 | Fig. 1Clinical features of the patient. (A) Knee contractures were noted bilaterally. (B) A big toe was hyperextensible. (C) Calcaneal bone was protuberant. |
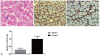 | Fig. 2Muscle pathology and quantification of the COL6A1 transcript. (A) H&E staining (×200) revealed round-shaped muscle fibers with marked size variations and surrounded by increased connective tissue. (B) Immunohistochemical staining (×200) of collagen VI was moderately reduced when compared with a disease control (C), but localized to both the sarcolemma and extracellular matrix. (D) The transcript level of COL6A1 as quantified by the quantitative reverse-transcription polymerase chain reaction was significantly decreased to 28.6% of the normal value. Bar = 50 µm. |
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D-1A1B03031011).
References
1. Camacho Vanegas O, Bertini E, Zhang RZ, Petrini S, Minosse C, Sabatelli P, et al. Ullrich scleroatonic muscular dystrophy is caused by recessive mutations in collagen type VI. Proc Natl Acad Sci U S A. 2001; 98:7516–7521.

2. Bönnemann CG. The collagen VI-related myopathies: muscle meets its matrix. Nat Rev Neurol. 2011; 7:379–390.

3. Briñas L, Richard P, Quijano-Roy S, Gartioux C, Ledeuil C, Lacène E, et al. Early onset collagen VI myopathies: genetic and clinical correlations. Ann Neurol. 2010; 68:511–520.

4. Yonekawa T, Nishino I. Ullrich congenital muscular dystrophy: clinicopathological features, natural history and pathomechanism(s). J Neurol Neurosurg Psychiatry. 2015; 86:280–287.

5. Yonekawa T, Komaki H, Okada M, Hayashi YK, Nonaka I, Sugai K, et al. Rapidly progressive scoliosis and respiratory deterioration in Ullrich congenital muscular dystrophy. J Neurol Neurosurg Psychiatry. 2013; 84:982–988.

6. Lee JH, Shin HY, Park HJ, Kim SH, Kim SM, Choi YC. Clinical, pathologic, and genetic features of collagen vi-related myopathy in Korea. J Clin Neurol. 2017; 13:331–339.

7. Butterfield RJ, Foley AR, Dastgir J, Asman S, Dunn DM, Zou Y, et al. Position of glycine substitutions in the triple helix of COL6A1, COL6A2, and COL6A3 is correlated with severity and mode of inheritance in collagen VI myopathies. Hum Mutat. 2013; 34:1558–1567.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download