초록
Skin biopsy and staining the specimens with immuno-reactive markers has been proven to be a useful method to demonstrate the pathologic status of small nerve fibers. Quantification of intraepidermal nerve fiber density using anti-protein gene product 9.5 antibody is a stan-dard method to diagnose small fiber neuropathy. Skin biopsy also makes it possible to differ-entiate the nerve fibers according to their function by using different markers. Quantification of dermal structures with different types of nerve fibers could reveal the pathophysiologic mechanism of the disease state.
Go to : 
REFERENCES
1.Wang L., Hilliges M., Jernberg T., Wiegleb-Edström D., Johansson O. Protein gene product 9.5-immunoreactive nerve fibres and cells in human skin. Cell Tissue Res. 1990. 261:25–33.

2.Kennedy WR., Wendelschafer-Crabb G. The innervation of human epidermis. J Neurol Sci. 1993. 115:184–190.

3.McCarthy BG., Hsieh ST., Stocks A., Hauer P., Macko C., Cornblath DR, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995. 45:1848–1855.

4.Lauria G., Cornblath DR., Johansson O., McArthur JC., Mellgren SI., Nolano M, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005. 12:747–758.

5.Lauria G., Hsieh ST., Johansson O., Kennedy WR., Leger JM., Mellgren SI, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010. 17:903–912. e44-49.
6.Mellgren SI., Nolano M., Sommer C. The cutaneous nerve biopsy: technical aspects, indications, and contribution. Handb Clin Neurol. 2013. 115:171–188.
7.Wang N., Gibbons CH. Skin biopsies in the assessment of the autonomic nervous system. Handb Clin Neurol. 2013. 117:371–378.

9.Wang N., Gibbons CH., Freeman R. Novel immunohistochemical techniques using discrete signal amplification systems for human cutaneous peripheral nerve fiber imaging. J Histochem Cytochem. 2011. 59:382–390.

10.Pittenger GL., Ray M., Burcus NI., McNulty P., Basta B., Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care. 2004. 27:1974–1979.

11.Chai J., Herrmann DN., Stanton M., Barbano RL., Logigian EL. Painful small-fiber neuropathy in Sjogren syndrome. Neurology. 2005. 65:925–927.

12.Kennedy WR., Wendelschafer-Crabb G. Utility of skin biopsy in diabetic neuropathy. Semin Neurol. 1996. 16:163–171.

13.Di Leo R., Nolano M., Boman H., Pierangeli G., Provitera V., Knappsk-og PM, et al. Central and peripheral autonomic failure in cold-in-duced sweating syndrome type 1. Neurology. 2010. 75:1567–1569.

14.Donadio V., Nolano M., Elam M., Montagna P., Provitera V., Bugiardi-ni E, et al. Anhidrosis in multiple system atrophy: a preganglionic sudomotor dysfunction? Mov Disord. 2008. 23:885–888.

15.Lauria G., Bakkers M., Schmitz C., Lombardi R., Penza P., Devigili G, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010. 15:202–207.

16.Devigili G., Tugnoli V., Penza P., Camozzi F., Lombardi R., Melli G, et al. The diagnostic criteria for small fibre neuropathy: from symp-toms to neuropathology. Brain. 2008. 131(Pt 7):1912–1925.

17.Nebuchennykh M., L⊘seth S., Jorde R., Mellgren SI. Idiopathic polyneuropathy and impaired glucose metabolism in a Norwegian patient series. Eur J Neurol. 2008. 15:810–816.

18.Nolano M., Provitera V., Donadio V., Stancanelli A., Saltalamacchia A., Caporaso G, et al. Ross syndrome: a lesson from a monozygotic twin pair. Neurology. 2013. 80:417–418.

19.Nolano M., Provitera V., Perretti A., Stancanelli A., Saltalamacchia AM., Donadio V, et al. Ross syndrome: a rare or a misknown dis-order of thermoregulation? A skin innervation study on 12 sub-jects. Brain. 2006. 129(Pt 2):2119–2131.

20.Smith AG., Ramachandran P., Tripp S., Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology. 2001. 57:1701–1704.

21.Sumner CJ., Sheth S., Griffin JW., Cornblath DR., Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003. 60:108–111.

22.Chao CC., Hsieh ST., Shun CT., Hsieh SC. Skin denervation and cutaneous vasculitis in eosinophilia-associated neuropathy. Arch Neurol. 2007. 64:959–965.

23.Tseng MT., Hsieh SC., Shun CT., Lee KL., Pan CL., Lin WM, et al. Skin denervation and cutaneous vasculitis in systemic lupus erythe-matosus. Brain. 2006. 129(Pt 4):977–985.

24.Nolano M., Manganelli F., Provitera V., Pisciotta C., Stancanelli A., Caporaso G, et al. Small nerve fiber involvement in CMT1A. Neurology. 2015. 84:407–414.

26.Nolano M., Provitera V., Caporaso G., Stancanelli A., Vitale DF., Santoro L. Quantification of pilomotor nerves: a new tool to evaluate autonomic involvement in diabetes. Neurology. 2010. 75:1089–1097.

27.Kennedy WR., Wendelschafer-Crabb G., Brelje TC. Innervation and vasculature of human sweat glands: an immunohistochemis-try-laser scanning confocal fluorescence microscopy study. J Neurosci. 1994. 14(11 Pt 2):6825–6833.

28.Dabby R., Djaldetti R., Shahmurov M., Treves TA., Gabai B., Melamed E, et al. Skin biopsy for assessment of autonomic denervation in Parkinson's disease. J Neural Transm (Vienna). 2006. 113:1169–1176.

29.Dabby R., Vaknine H., Gilad R., Djaldetti R., Sadeh M. Evaluation of cutaneous autonomic innervation in idiopathic sensory small-fi-ber neuropathy. J Peripher Nerv Syst. 2007. 12:98–101.

30.Gibbons CH., Illigens BM., Wang N., Freeman R. Quantification of sudomotor innervation: a comparison of three methods. Muscle Nerve. 2010. 42:112–119.

31.Gibbons CH., Illigens BM., Wang N., Freeman R. Quantification of sweat gland innervation: a clinical-pathologic correlation. Neurology. 2009. 72:1479–1486.

32.Tesfaye S., Boulton AJ., Dyck PJ., Freeman R., Horowitz M., Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010. 33:2285–2293.

33.Beach TG., White CL., Hamilton RL., Duda JE., Iwatsubo T., Dick-son DW, et al. Evaluation of alpha-synuclein immunohisto-chemical methods used by invited experts. Acta Neuropathol. 2008. 116:277–288.
34.Shannon KM., Keshavarzian A., Mutlu E., Dodiya HB., Daian D., Jaglin JA, et al. Alpha-synuclein in colonic submucosa in early untreated Parkinson's disease. Mov Disord. 2012. 27:709–715.

35.Pouclet H., Lebouvier T., Coron E., des Varannes SB., Rouaud T., Roy M, et al. A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson's disease. Neurobiol Dis. 2012. 45:305–309.

36.Orimo S., Uchihara T., Nakamura A., Mori F., Kakita A., Wakabayashi K, et al. Axonal alpha-synuclein aggregates herald centripetal de-generation of cardiac sympathetic nerve in Parkinson's disease. Brain. 2008. 131(Pt 3):642–650.
37.Nolano M., Provitera V., Estraneo A., Selim MM., Caporaso G., Stancanelli A, et al. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain. 2008. 131(Pt 7):1903–1911.

38.Wang N., Gibbons CH., Lafo J., Freeman R. α-Synuclein in cutaneous autonomic nerves. Neurology. 2013. 81:1604–1610.

Go to : 
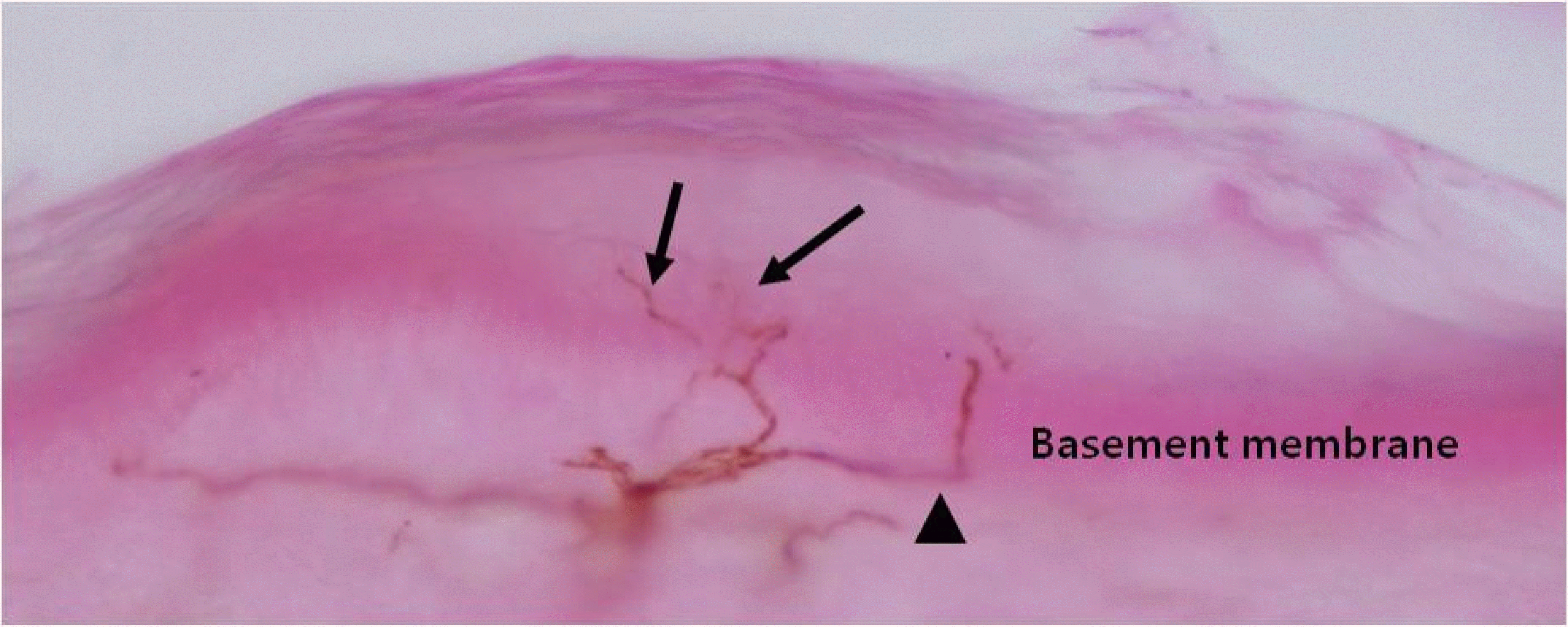 | Fig. 1.Nerve fibers of epidermis and superficial dermis. Epidermal nerve fibers (arrows) originate from the subepidermal neural plexus (arrowhead) and travel vertically to the epidermal surface. (anti-PGP 9.5 antibody for nerve fiber). |
 | Fig. 2.Dermal structures stained with anti-PGP 9.5 antibodies for nerve fibers (red) and anti-CD31 antibodies for blood vessels (green). (A, C) Deep dermal vessels are densely surrounded by innervating nerve fibers. (B) The nerve fibers innervating arrector pili muscle run parallel with muscle fibers. Intervening blood vessels were also showed. (D) Sweat gland and intervening capillaries. Sweat gland is innervated densely and complicatedly. |
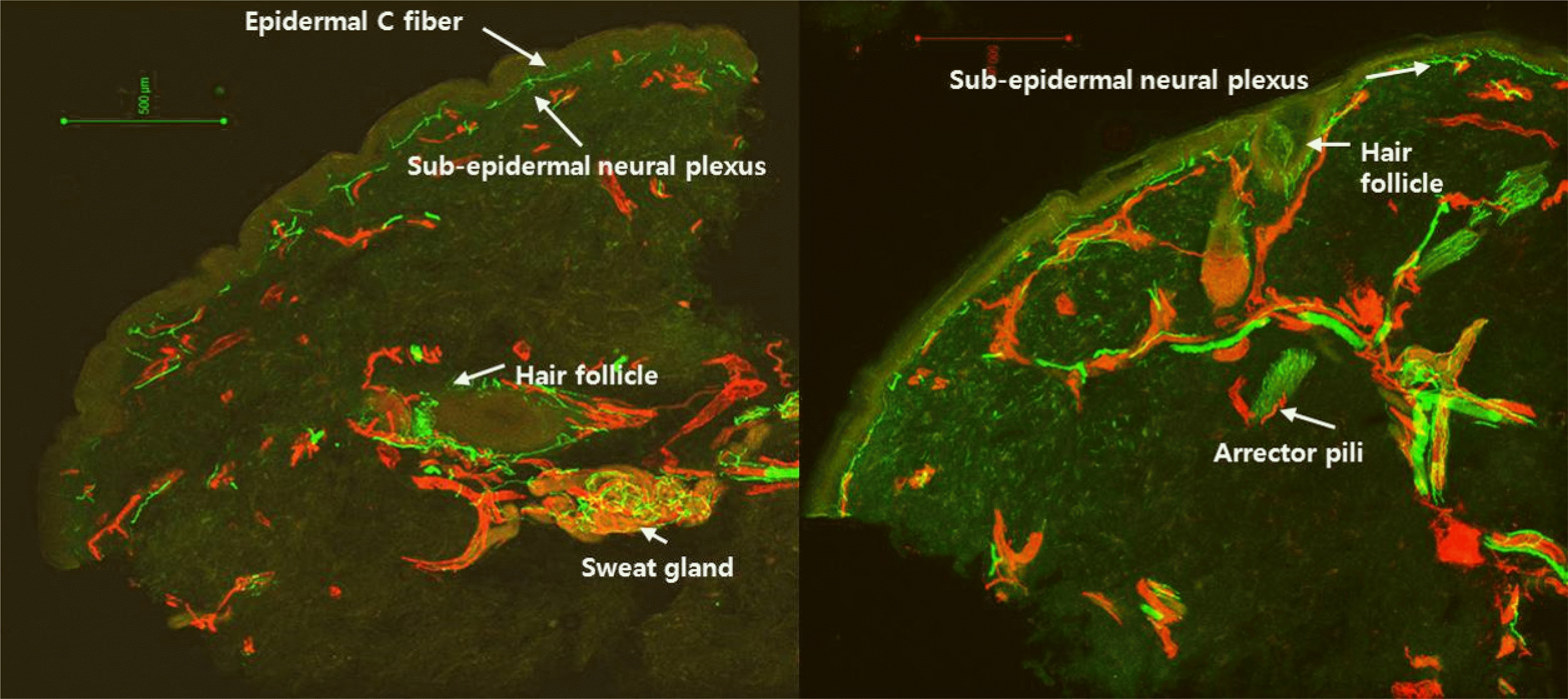 | Fig. 3.Samples of stained skin biopsy tissue. Epidermal C fiber and subepidermal neural plexus are located at the superficial dermis. Hair follicle travels from the deep dermis to the superficial dermis and arrector pili muscles are located near the hair follicle. Sweat glands are located in the deep dermis and innervated densely (anti-PGP 9.5 antibodies for nerve fibers [green] and anti-CD31 antibodies for endothelia [red]). |
 | Fig. 4.Example of images obtained by immunohistochemical staining. (A-C) A sweat gland. Endothelia of capillaries are stained with anti-CD31 antibody in green (A). Nerve fibers innervating sweat gland are stained with anti-PGP 9.5 antibody in red (B). Merged image (C). (DF) A sweat gland and endothelia (anti-CD31 antibody, blue). Sympathetic cholinergic fibers are stained with anti-VIP antibody in green (D) and sympathetic adrenergic fibers are stained with anti-TH antibody in red (E). Merged image (F). (G-I) Cutaneous blood vessels. Endothelia are stained wit an-ti-CD31 antibody in red and nerve fibers are stained with anti-PGP 9.5 antibody in green. (J-L) Arrector pili muscles are innervated with sympathetic adrenergic fibers (anti-TH antibody, J) and sympathetic cholinergic fibers (anti-VIP antibody, K). Merged image (L). Originally adapted from Wang et al.7
|
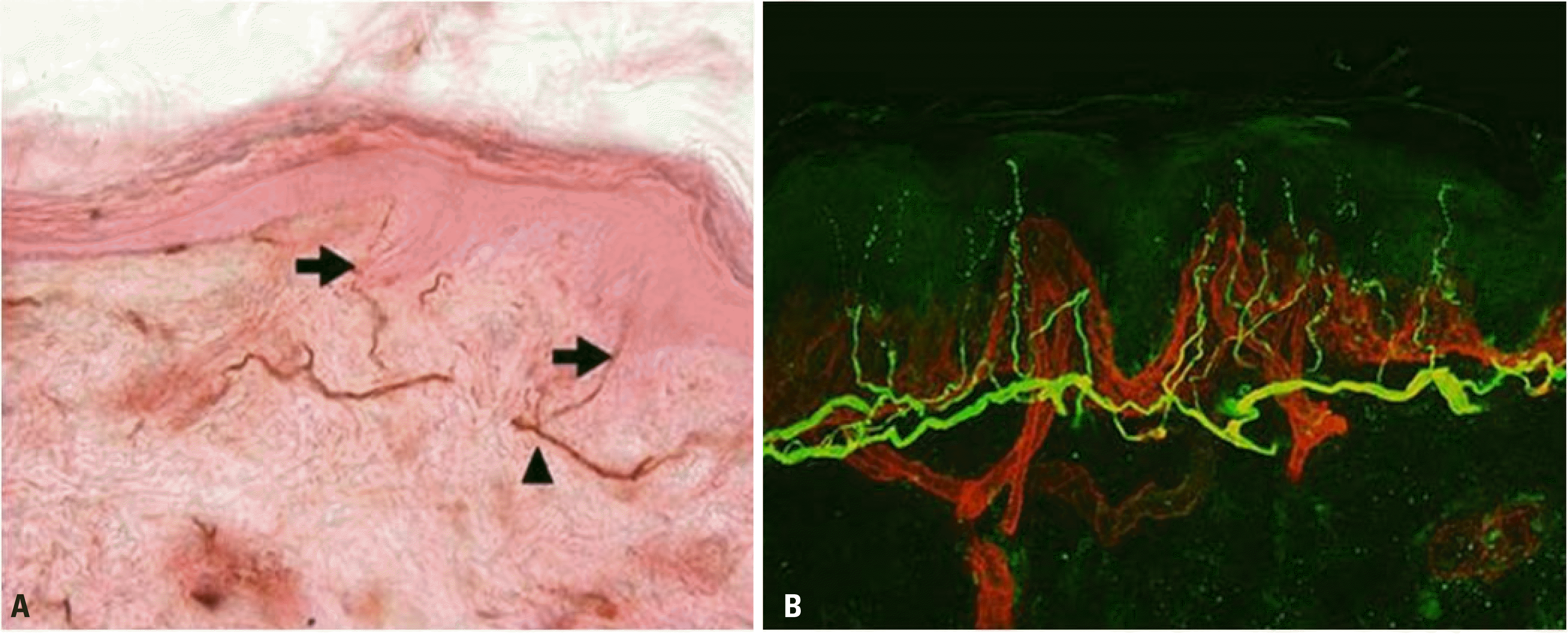 | Fig. 5.Dermal and epidermal nerve fibers stained with anti-PGP 9.5 antibodies. (A) Bright-field microscopy finding. Intraepidermal nerve fibers (arrows) cross from the sunepidermal neural plexus (arrowhead) to the epidermis. (B) Confocal microscopy showing nerve fibers (in green) and blood vessels and basement membrane (in red). |
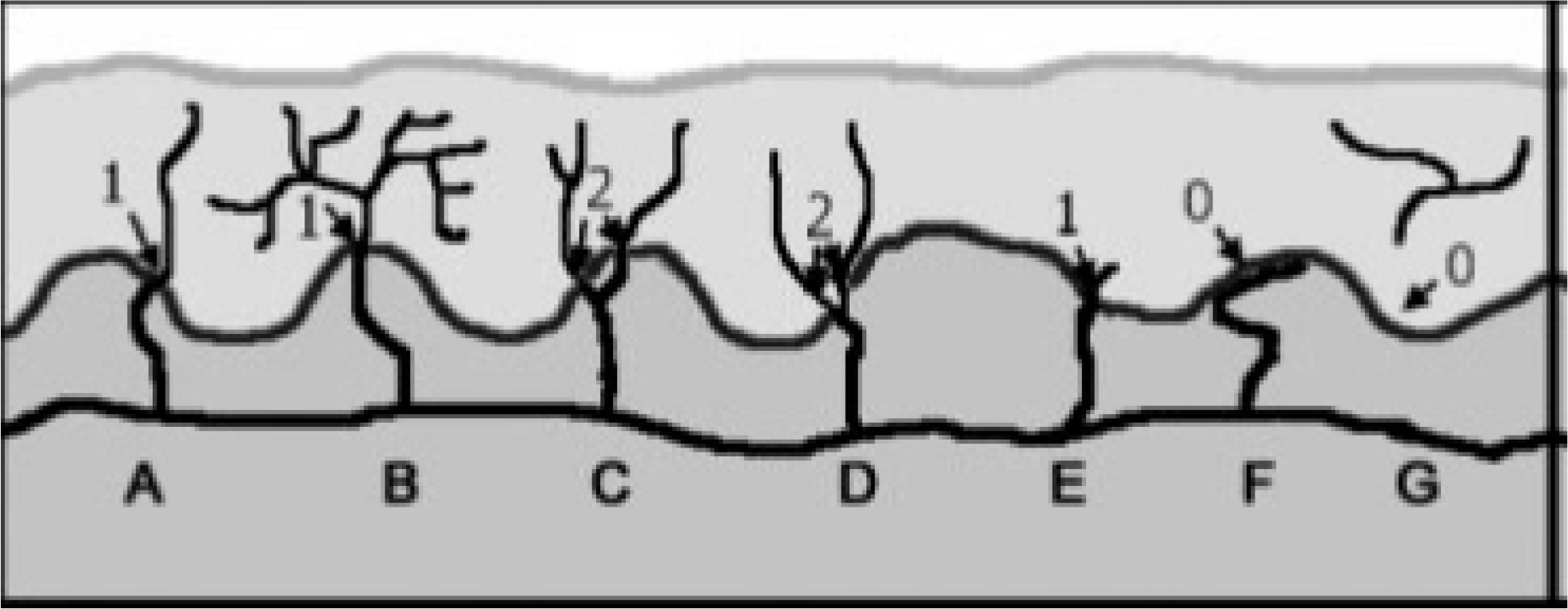 | Fig. 6.Intraepidermal nerve fiber counting rule. Diagram of skin in-nervations: nerves (black), basement membrane (dark grey), dermis (medium grey), and epidermis (light grey). Nerve fibers which cross the basement membrane are only counted as one nerve fiber. Nerve fibers which branches after crossing the basement membrane or which re-sides only in the epidermis should be excluded when count the nerve fibers. The epidermal nerve fiber branches before crossing the basement membrane, it should be counted as two fibers. Originally adapted 4
|
Table 1.
Normal values of intraepidermal nerve fiber density at the ankle




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download