초록
Background
We evaluated whether eye movements could reflect the efficacy of antiepileptic drugs in patients with epilepsy.
Methods
Thirty patients with epilepsy of unknown cause as well as age- and sex-matched normal controls were enrolled in this study. We divided the patients into drug-controlled ep-ilepsy (n = 22) and drug-resistant epilepsy (n = 8) groups according to their seizure controls. We analyzed the differences in the parameters of the eye movements in these two groups compared with normal controls using video-based electro-oculography. In addition, we in-vestigated the differences in the cerebellar volumes of these two groups using whole-brain T1-weighted images.
Results
The latency and accuracy of saccade in patients with epilepsy were significantly different from normal controls, but they were not different between patients with drug-con-trolled epilepsy and drug-resistant epilepsy. However, the gain of pursuit was significantly decreased in patients with drug-resistant epilepsy compared with normal controls (p = 0.0010), whereas it was not different between patients with drug-controlled epilepsy and normal controls (p = 0.9646). In addition, the patients with drug-resistant epilepsy had lower cerebellar volumes than normal controls (p = 0.0052), whereas the cerebellar volumes in patients with drug-controlled epilepsy were not different from normal controls (p = 0.5050).
REFERENCES
1.Ngugi AK., Kariuki SM., Bottomley C., Kleinschmidt I., Sander JW., Newton CR. Incidence of epilepsy: a systematic review and me-ta-analysis. Neurology. 2011. 77:1005–1012.

2.Rossetti AO., Villemure JG., Seeck M., Prilipko O., Despland PA., Jallon P. Current epilepsy treatment in adults. Rev Med Suisse. 2005. 1:1220, 1222, 1224-1226.
3.Brodie MJ., Barry SJ., Bamagous GA., Norrie JD., Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012. 78:1548–1554.

4.Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia. 1998. 39:799–803.
5.Glauser T., Ben-Menachem E., Bourgeois B., Cnaan A., Chadwick D., Guerreiro C, et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006. 47:1094–1120.

6.Glauser T., Ben-Menachem E., Bourgeois B., Cnaan A., Guerreiro C., Kälviäinen R, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013. 54:551–563.

7.Siddiqui A., Kerb R., Weale ME., Brinkmann U., Smith A., Goldstein DB, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003. 348:1442–1448.
8.Kwan P., Poon WS., Ng HK., Kang DE., Wong V., Ng PW, et al. Mul-tidrug resistance in epilepsy and polymorphisms in the volt-age-gated sodium channel genes SCN1A, SCN2A, and SCN3A: correlation among phenotype, genotype, and mRNA expression. Pharmacogenet Genomics. 2008. 18:989–998.

9.Amstutz U., Shear NH., Rieder MJ., Hwang S., Fung V., Nakamura H, et al. Recommendations for HLA-B*15:02 and HLA-A*31:01 genetic testing to reduce the risk of carbamazepine-induced hypersensitivity reactions. Epilepsia. 2014. 55:496–506.

10.Park KM., Shin KJ., Ha SY., Park J., Kim SE., Kim HC, et al. Can the adverse effects of antiepileptic drugs be detected in saccadic eye movements? Seizure. 2015. 25:33–36.

11.Berg AT., Berkovic SF., Brodie MJ., Buchhalter J., Cross JH., van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010. 51:676–685.

12.Kwan P., Arzimanoglou A., Berg AT., Brodie MJ., Allen Hauser W., Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010. 51:1069–1077.

13.Deckers CL., Hekster YA., Keyser A., Meinardi H., Renier WO. Reap-praisal of polytherapy in epilepsy: a critical review of drug load and adverse effects. Epilepsia. 1997. 38:570–575.

14.Leigh RJ., Zee DS. The neurology of eye movements. 4th ed.New York: Oxford University Press;2015. 199.
15.Fischl B., Salat DH., Busa E., Albert M., Dieterich M., Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroana-tomical structures in the human brain. Neuron. 2002. 33:341–355.
16.Fischl B., van der Kouwe A., Destrieux C., Halgren E., Ségonne F., Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004. 14:11–22.

17.Krauzlis RJ. The control of voluntary eye movements: new per-spectives. Neuroscientist. 2005. 11:124–137.

19.Suzuki DA., Yamada T., Hoedema R., Yee RD. Smooth-pursuit eye-movement deficits with chemical lesions in macaque nucle-us reticularis tegmenti pontis. J Neurophysiol. 1999. 82:1178–1186.

20.Ono S. The neuronal basis of on-line visual control in smooth pursuit eye movements. Vision Res. 2015. 110(Pt B):257–264.

21.Baier B., Stoeter P., Dieterich M. Anatomical correlates of ocular motor deficits in cerebellar lesions. Brain. 2009. 132(Pt 8):2114–2124.

22.Zee DS., Yamazaki A., Butler PH., Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981. 46:878–899.

23.Brodal A. Cerebrocerebellar pathways. Anatomical data and some functional implications. Acta Neurol Scand Suppl. 1972. 51:153–195.
25.Davis R., Emmonds SE. Cerebellar stimulation for seizure control: 17-year study. Stereotact Funct Neurosurg. 1992. 58:200–208.

26.Specht U., May T., Schulz R., Rohde M., Ebner A., Schmidt RC, et al. Cerebellar atrophy and prognosis after temporal lobe resection. J Neurol Neurosurg Psychiatry. 1997. 62:501–506.

27.Hagemann G., Lemieux L., Free SL., Krakow K., Everitt AD., Kendall BE, et al. Cerebellar volumes in newly diagnosed and chronic epilepsy. J Neurol. 2002. 249:1651–1658.

Fig. 1.
Differences in the cerebellar volumes in patients with epilepsy and normal controls. The results reveal that the cerebellar volumes in patients with drug-resistant epilepsy are significantly smaller than those in normal controls (p = 0.0180), whereas the cerebellar volumes are not different between patients with drug-controlled epilepsy and normal controls (p = 0.5050).
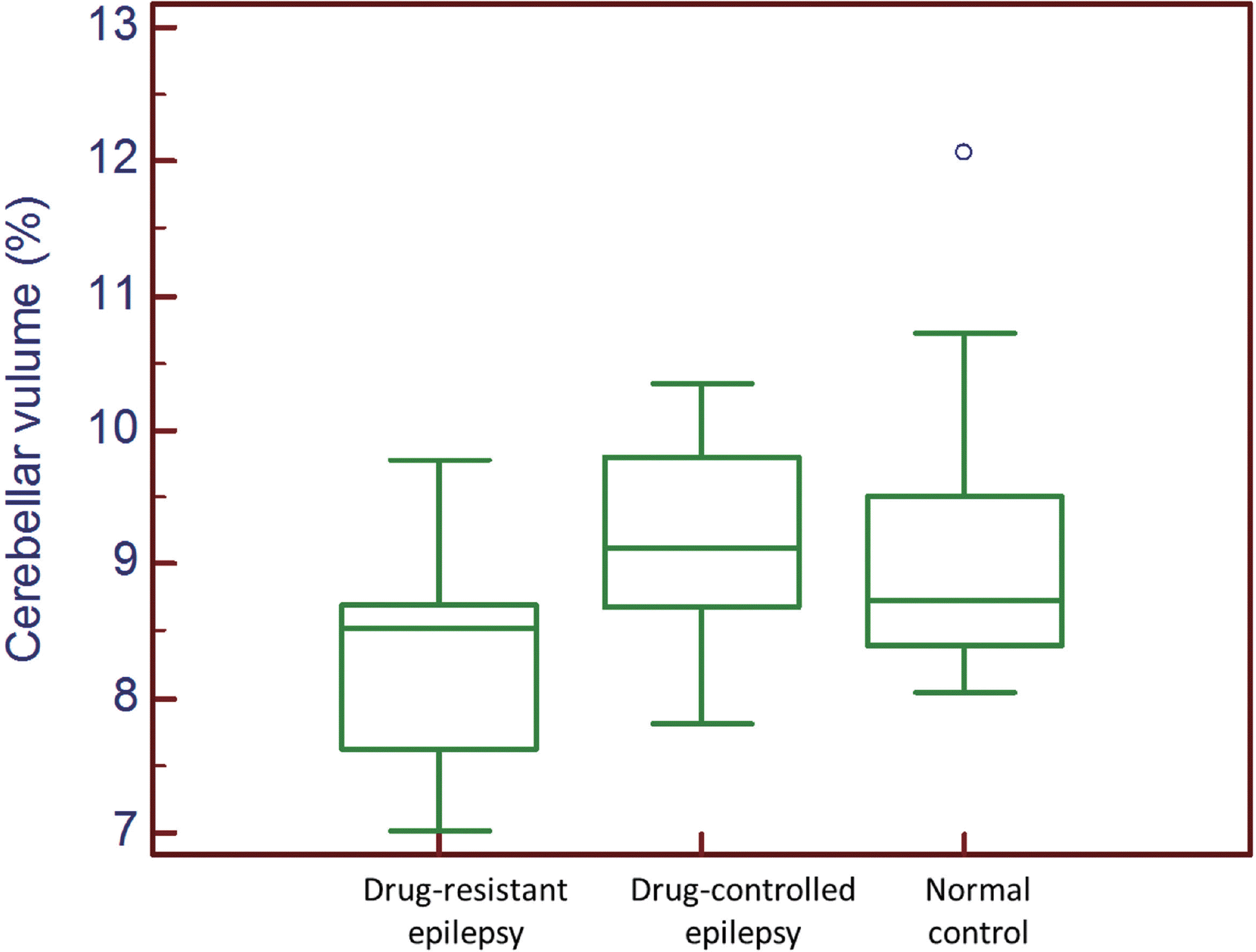
Table 1.
Differences in the demographic and clinical characteristics between patients with drug-resistant epilepsy and drug-controlled epilepsy
Table 2.
Differences in the parameters of video-based electro-oculography between patients with drug-resistant epilepsy and drug-controlled epilepsy compared with normal controls




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download