Indicators that may be used to predict neurological recovery post-cardiopulmonary arrest include brainstem reflexes, myoclonus status epilepticus, somatosensory evoked potentials (SEP), electroencephalography (EEG), and serum neuron specific enolase (NSE).1 Among these, SEP is a useful test which can be easily performed in the intensive care unit (ICU), with the absence of bilateral N20 responses being a poor prognostic sign known to have a false positive rate close to 0.1
We report a case of myoclonus status epilepticus recurring in a patient with amyotrophic lateral sclerosis while performing SEP study to evaluate the degree of hypoxic injury, and investigate the risk factors and pathophysiology of this condition.
CASE
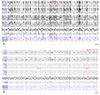
A 77-year-old male was resuscitated after 6 minutes of respiratory arrest. He started having bilateral upper extremity weakness 4 years ago and was diagnosed with amyotrophic lateral sclerosis, and developed dyspnea a year ago. An hour post-arrest he developed generalized myoclonic jerks which would occur every few seconds, which resolved gradually over the course of 2 days. He was treated with intravenous midazolam infusion (1 mg/kg/hour) and clonazepam via nasogastric tube (9 mg/24 hour), and midazolam was discontinued after the myoclonic jerks had resolved. EEG at that time showed generalized slowing at 1.5–2 Hz between the muscle artifact due to the myoclonus (Fig. 1). Serum NSE levels 30 hours post-event were within normal range at 13.02 ng/mL, and diffusion MRI on day 3 did not demonstrate any acute changes. EEG showed generalized continuous small spikes at frequencies above 1 Hz with maximum amplitudes in bilateral frontocentral areas, with rare gross head movements, making it challenging to determine as to whether this was artifact associated with fine myoclonus of the head or true potentials originating from the brain itself (Fig. 2). Median nerve SEP testing was performed on day 5 while the patient was still in a comatose state. When both wrists were electrically stimulated, normal latencies and waveforms of evoked potentials were observed from scalp leads C3, and C4. After median nerve SEP testing the patient developed recurrent myoclonus in the face and extremities, some of which were spontaneous and others triggered by passive movements or somatosensory stimuli. Due to frequent myoclonic artifact the background was difficult to assess (Fig. 3A), but with intravenous vecuronium the myoclonus subsided and spike and slow-wave complexes occurring at least 2 Hz with phase reversal at C3, and C4 were observed (Fig. 3B), which could be suppressed with intravenous lorazepam. Levetiracetam (1,000 mg/24 hour) was subsequently administered but multifocal myoclonus continued to recur, which resolved 10 days later. The patient expired 2 months later due to systemic infection without any recovery of consciousness.
DISCUSSION
Various types of myoclonus exist in terms of distribution, source, and etiology. Not uncommonly does stimulus-sensitive or reflex myoclonus occur, which is provoked by different stimuli. The myoclonus after SEP testing in this case may be classified as multifocal myoclonus in terms of distribution, as cortical or epileptic myoclonus associated with fairly focal epileptiform discharges on EEG, or reflex myoclonus provoked by somatosensory stimuli.23
In general postanoxic myoclonus status epilepticus, which occurs in the acute phase several hours post-anoxic injury, spontaneously resolves within several days, and is known to be a poor prognostic factor implying diffuse cerebral injury.1 Hallett et al. suggested that the source of acute postanoxic myoclonus is the medulla oblongata based on the fact that the trapezius was activated prior to the scalp EEG activity and orbicularis oculi, masseter, and arm muscles activity later when performing surface electromyography on various muscles innervated by cranial nerves during myoclonus among patients who woke up 2 weeks post-cardiopulmonary arrest.4 In cases of chronic postanoxic myoclonus, otherwise known as Lance-Adams syndrome, the source is considered to be cortex for the most part, the rationale being that EEG activity is observed to precede myoclonus on EEG back-averaging and at times with giant SEPs.5 However, generalized spikes may be induced on EEG by stimulus in addition myoclonus in a number of cases of stimulus sensitive postanoxic myoclonus during the acute stages,6 hence this may be considered to be a type of reflex epilepsy involving the cerebral cortex. The ictal EEG of acute postanoxic myoclonus has been reported to manifest in various patterns. Burst suppression or generalized periodic epileptiform discharges are common, but diffuse slowing or low voltage EEG may also be seen, and when burst suppression or generalized periodic epileptiform discharges are detected the myoclonus may or may not be time-locked to the bursts or spikes. This suggests that various mechanisms for postanoxic myoclonus status epilepticus may be involved,789 which may also be a result of differences in location and degree of anoxic injury.
This was an unprecedented case of documented spikes with a central focus in acute postanoxic myoclonus. There have been cases of vertex, central, and frontal-dominant generalized spikes,10 and there might be a lack of reports due to difficulty ascertaining these discharges in the midst of myoclonic artifacts. Acute postanoxic myoclonus may either occur spontaneously or with sensory stimuli, somatosensory stimuli being the most common. This suggests that the somatosensory cortex might be the most vulnerable to hypoxic injury. Even in other causes of myoclonus the EEG activity observed with back-averaging was found to originate from the sensorimotor cortex much like SEPs.3 This patient was suffering from amyotrophic lateral sclerosis, and we assumed that the underlying cortical neuronal degeneration might have been more prone to irritability after an anoxic insult, especially in the pericentral areas. However, myoclonus or seizures are not more common spontaneously in amyotrophic lateral sclerosis.
Perhaps the main topic of discussion is whether or not the postanoxic myoclonus status epilepticus after SEP testing was truly due to the SEP study. To date there have been no studies on the potential association between SEP testing and postanoxic myoclonus status epilepticus. Stimulus-sensitive myoclonus frequently occurs post-anoxia, and considering that hyperexcitability of the sensorimotor cortex is frequently observed, it is possible that the repetitive electrical stimulation of sensory nerves might have induced myoclonic status. One cannot exclude the possibility of this being the nature course of postanoxic myoclonus or the recurrence of myoclonus which improved after decreasing the dose of midazolam, but postanoxic myoclonus has been reported to spontaneously resolve within 5 days in most cases.8 The initial myoclonus seen in this patient resolved within 2 days, which is consistent with its common clinical course, but is unusual for postanoxic myoclonus to recur 5 days post-arrest and persist for 10 days. The majority of cases of chronic postanoxic myoclonus after recovery from anoxia have been reported to demonstrate myoclonus in the acute phase, and in this case the patient might have progressed to chronic postanoxic myoclonus with time. However, neurologically this patient did not recover, demonstrating repeated resolution of myoclonus within 10 days, which differs from chronic postanoxic myoclonus in general.
SEP testing may provoke post-anoxic myoclonic status epilepticus. Future studies are required to assess the frequency and risk factors, whether or not SEP is safe to perform in patients who have demonstrated postanoxic myoclonus, or if the underlying condition of amyotrophic lateral sclerosis had attributed to this phenomenon or not.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download