Abstract
Purpose
The management of primary tumors in patients with stage IV colorectal cancer remains unclear. This meta-analysis evaluated the survival benefits of primary tumor resection (PTR) in patients with unresectable stage IV colorectal cancer in the era of modern chemotherapy.
Methods
Multiple comprehensive databases were searched for studies comparing survival outcomes in patients with metastatic colorectal cancer who did and did not undergo PTR. Outcome data were pooled, and overall effect size was calculated using random effect models.
Results
Seventeen nonrandomized studies involving 18,863 patients met the inclusion criteria. Meta-analysis showed that PTR significantly improved overall survival (hazard ratio [HR], 0.63; 95% confidence interval [CI], 0.56–0.71; P < 0.001) and progression free survival (HR, 0.76; 95% CI, 0.67–0.87; P < 0.001). Subgroup analyses and sensitivity analyses, performed by predefined methods, also indicated that PTR improved overall patient survival.
Approximately 20%–25% of patients with colorectal cancer present with metastases at the time of diagnosis. Most patients with metastatic colorectal cancer have unresectable disease, but may benefit from chemotherapy or primary tumor resection (PTR) with palliative intent followed by systemic chemotherapy [1]. PTR may improve quality of life and may prevent or ameliorate complications caused by growth of the primary tumor, such as obstruction, perforation or bleeding [2345]. These complications require emergency surgery, which is associated with high morbidity and mortality rates and less favorable long-term outcomes. Despite the benefits of PTR, it delays the start of systemic chemotherapy, which also provides survival advantages [67]. Systemic chemotherapy regimens that contain agents such as irinotecan, oxaliplatin, and targeted agents have improved the prognosis of these patients. Administration of systemic chemotherapy to most patients who lack symptoms related to primary tumors may be sufficient to control the asymptomatic primary lesions, as chemotherapy can shrink tumors and/or control tumor spread [891011]. Moreover, PTR may lead to postoperative morbidity and mortality, making resection questionable even in patients with asymptomatic tumors.
It is therefore unclear whether PTR with palliative intent is safe or provides actual survival benefit. Although three previous meta-analyses reported oncologic outcomes of PTR [21213], 2 of those analyses assessed overall survival (OS) alone, and did not consider the survival outcomes of subgroups. Furthermore, all of these analyses included studies published prior to the introduction of chemotherapeutic regimens that contained irinotecan or oxaliplatin. The current meta-analysis therefore evaluated the survival benefits of PTR in patients with unresectable metastatic colorectal cancer in the era of modern chemotherapy.
This meta-analysis followed the recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [14]. Multiple comprehensive databases were searched for studies that assessed the oncologic outcomes of PTR in patients with unresectable metastatic colorectal cancer. The study protocol was based on Cochrane Review Methods [15].
PubMed (January 1, 1976 to November 23, 2016), Embase (January 1, 1985 to November 23, 2016), and the Cochrane Central Register of Controlled Trials (CENTRAL; January 1, 1987 to November 23, 2016) were searched. There were no restrictions on year or language of publication. The search terms used were “colorectal cancer,” “metastatic,” “stage IV,” “palliative resection,” and “survival.” After the initial electronic search, articles were manually searched to identify additional studies. All articles were assessed individually before inclusion.
Article titles and abstracts were screened, and full texts were reviewed independently by 2 reviewers (GWH and MRL), based on the selection criteria. Discrepancies were resolved by discussion between these reviewers.
Included studies assessed the survival outcomes, including OS and progression-free survival (PFS), of patients with metastatic colorectal cancer who did and did not undergo PTR. Studies were excluded if they: (1) assessed patients who had tumor pathology other than adenocarcinoma; (2) assessed patients who were not treated with modern cytotoxic agents, such as irinotecan or oxaliplatin, or were diagnosed with metastatic colorectal cancer before 2000, the beginning of the modern chemotherapy era; (3) assessed patients who underwent simultaneous or subsequent metastasectomy; (4) assessed only specific groups of patients (e.g., elderly or obese patients); (5) had no extractable data and the authors could not be reached to provide additional information; (6) were case series with fewer than 10 patients; and (7) were not published in English.
All eligible studies were reviewed, and all relevant data were extracted independently by 2 reviewers using a predefined data extraction form. The variables recorded were: (1) basic publication information, including name of the first author, year of publication, and number of patients; (2) demographic, clinical, and treatment characteristics of the patients; and (3) patient outcomes (OS and PFS).
The methodological quality of the included studies was assessed using the Newcastle-Ottawa quality scale (NOS), which allocates a maximum of 9 points to each study; a score ≥ 6 indicated high quality [16]. The quality of included studies was determined by examining three factors: patient selection, comparability of the study groups and assessment of outcomes.
The meta-analysis determined the hazard ratio (HR) with its variance and 95% confidence interval (CI). The presence and extent of heterogeneity were assessed using the Q test and I2 index, respectively, with a P-value less than 0.1 considered statistically significant [17]. The DerSimonian-Laird random effects model (REM) was used for pooling data in anticipation of cross-study heterogeneity [18]. If sufficient data were available, planned subgroup analyses were performed to evaluate oncologic effects of PTR. Sensitivity analyses were also performed to assess the robustness of the meta-analysis findings [1920]. Publication bias was assessed by visually inspecting funnel plots of the outcome, and using the Egger weighted linear regression test, in which a P-value less than 0.1 was considered significant [2122].
All data were analyzed using Review Manager (RevMan 5.3, Cochrane Collaboration, Oxford, UK) and Comprehensive Meta-Analysis ver. 3 (Englewood, NJ, USA).
The predefined searching strategy and manual searching identified 13,577 potentially relevant articles; of these, 2,436 articles were excluded because they were duplicates and 11,070 were excluded because their titles and abstracts did not fulfill the selection criteria. After full text review of the remaining 71 articles, 54 were excluded due to the exclusion criteria of these studies. Therefore, a total of 17 nonrandomized studies were deemed suitable for inclusion (Fig. 1). These studies examined a total of 18,863 patients, 9,575 of whom underwent PTR without metastasectomy. All 17 studies evaluated OS [2324252627282930313233343536373839], whereas only two evaluated PFS [2435]. Three studies examined patients with colon cancer exclusively [283238], one examined patients with rectal cancer exclusively [25], and 13 examined patients with colorectal cancer [23242627293031333435363739]; in 3 of the latter studies, data on patients with colon cancer could be separated from data on patients with rectal cancer [343739]. Thirteen studies evaluated patients who received targeted chemotherapy [23242627282930313233343539]. In eight studies, all patients who did not undergo PTR received systemic chemotherapy [2324283032333536]. Four studies evaluated oncologic outcomes using large databases [34363738]. Seven studies assessed HR for OS using propensity score analysis [27293132363738].
Analysis of oncologic outcomes after PTR indicated that all 17 studies, involving 18,863 patients, reported data on OS. Pooled analysis showed that PTR increased OS rate (HR, 0.63; 95% CI, 0.56–0.71; I2 = 83%) (Fig. 2). Two studies, involving 1,038 patients, reported PFS after PTR, with PTR found to increase rate (HR, 0.76; 95% CI, 0.67–0.87, I2 = 0%) (Fig. 3).
The effects of PTR on OS were also determined in patient subgroups, depending on chemotherapy regimens and tumor location. These subgroups included (1) patients who received targeted chemotherapy, (2) all patients in the non-PTR group underwent chemotherapy, and (3) patients with colon or rectal cancer alone. OS was increased in groups of patients who received chemotherapy that included a targeted agent (bevacizumab, cetuximab, or panitumumab) (HR, 0.65; 95% CI, 0.58–0.73; I2 = 37%); patients in the non-PTR group who received chemotherapy (HR, 0.63; 95% CI, 0.57–0.70; I2 = 19%) and patients with colon cancer alone who underwent PTR (HR, 0.63; 95% CI, 0.48–0.85; I2 = 90%). Because only one study reported OS in patients with rectal cancer alone, subgroup analysis was not performed.
Sensitivity analyses, performed using predefined methods, indicated the robustness of all OS results of this meta-analysis. Reanalysis of the data using an alternative statistical effects model (HR, 0.54; 95% CI, 0.52–0.56; I2 = 83%), analysis of high-quality studies (NOS ≥ 6) (HR, 0.62; 95% CI, 0.54–0.70; I2 = 81%), analysis of studies reported by surgeons (HR, 0.62; 95% CI, 0.51–0.75; I2 = 89%), analysis of studies reported by medical oncologist (HR, 0.64; 95% CI, 0.57–0.72; I2 = 36%), and analysis of studies using large databases (HR, 0.64; 95% CI, 0.50–0.83; I2 = 96%) all showed that PTR was associated with increased OS. Finally, analysis of studies using HRs for OS from propensity score analysis showed that PTR was associated with increased OS (HR, 0.64; 95% CI, 0.52–0.80; I2 = 88%). Table 3 summarizes the results of these subgroup and sensitivity analyses
Publication bias was analyzed using the Egger weighted linear regression test, which assesses the asymmetry of funnel plots. The funnel plot for OS (P = 0.01) was found to be asymmetric, indicating publication bias (Fig. 4).
To our knowledge, this is the first meta-analysis to assess the oncologic effect of PTR for patients diagnosed with unresectable stage IV colorectal cancer in the era of modern chemotherapy. This meta-analysis found that PTR was associated with increased OS in patients with unresectable metastatic colorectal cancer. This result is similar to that of previous meta-analyses [21213]. However, these previous analyses included studies that evaluated patients diagnosed with colorectal cancer before the introduction of modern chemotherapy regimens which led to a major transition in the treatment of metastatic colorectal cancer [40]. Irinotecan was introduced in 1996, and it was reported that irinotecan provided survival benefits in patients with metastatic colorectal cancer in 2000 [41]. Therefore, we chose a time period of diagnosis beginning in 2000. The number of new chemotherapeutic agents has increased significantly over the past decade, with these new agents playing an important role in the treatment of metastatic colorectal cancer. Therefore, the present meta-analysis included studies that assessed patients treated with modern cytotoxic agents, such as irinotecan or oxaliplatin, or assessed patients diagnosed with metastatic colorectal cancer after 2000.
The present study provides a more detailed assessment of oncologic outcomes, using subgroup and sensitivity analyses, than previous studies. We found that PTR was associated with increased PFS. PTR was also found to be associated with increased OS from the analysis of studies including patients who received target agents, and from the analysis of studies in which all patients in non-PTR group received chemotherapy. Despite selection bias, these results suggest that PTR may be an independent prognostic factor for OS, similar to a study that reported that PTR itself was an independent predictor of response to bevacizumab [42]. PTR may reduce the concentrations of tumor-derived protumorigenic chemotactic cytokines that regulate cancer metastasis [4344]. Primary tumors may have unique attributes that support tumor progression. Primary tumors frequently exhibit higher genetic variability than metastases and, therefore, may have greater capacity to generate new metastatic clones resistant to ongoing treatments [404546]. Additionally, we tried to separately analyze patients with colon cancer and rectal cancer, because the natural history, complications and therapeutic options differ in these 2 groups. We found that PTR was associated with increased OS in patients with colon cancer. However, it was impossible to analyze patients with rectal cancer, because only one of the 17 included studies separately analyzed patients with rectal cancer.
This meta-analysis had several limitations. There was potential heterogeneity among the included studies, despite our use of definite exclusion criteria and our performance of subgroup and sensitivity analyses. Because all included studies were nonrandomized, selection bias was likely. Patient performance status, presence of symptoms, tumor burden, and various surgical techniques may have influenced the oncologic outcomes of PTR. Moreover, most studies included in this analysis did not specify reasons or criteria for nonresection. The included studies were retrospective in design, suggesting a possible bias in decision-making for individual patients, which may have influenced oncologic outcomes. Although the factors that may have influenced the selection of patients for surgery remain unclear, patients who were younger in age and had fewer comorbidities and metastases were more likely to undergo PTR, whereas patients who were older, sicker and had many metastatic foci were less likely to undergo surgery. Finally, Publication bias was found in the analysis of OS. However, using the Trim-and-Fill method, under both of the fixed effects model and the REM for the outcomes, our results were not affected by this bias.
In conclusion, surgical resection of the primary tumor without metastasectomy may have survival benefits in patients with unresectable stage IV colorectal cancer. Continued advances in modern chemotherapy and appropriate local treatments including PTR would improve survival outcomes. Randomized controlled trials are needed to determine the optimal treatment for these patients.
Figures and Tables
Fig. 1
Flow chart of the literature search according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.
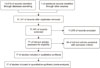
Fig. 2
Forest plot and meta-analysis of the effects of primary tumor resection on overall survival. SE, standard error; CI, confidence interval; df, degree of freedom.

Fig. 3
Forest plot and meta-analysis of the effects of primary tumor resection on progression-free survival. SE, standard error; CI, confidence interval; df, degree of freedom.

Fig. 4
Funnel plots of included studies in the analysis for the effects of primary tumor resection on overall survival. SE, standard error.

ACKNOWLEDGMENTS
The authors thank the reviewers for their extremely thorough and helpful reviews and suggestions.
References
1. Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol. 2005; 12:637–645.

2. Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg. 2010; 34:797–807.

3. Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg. 2003; 196:722–728.

4. Cummins ER, Vick KD, Poole GV. Incurable colorectal carcinoma: the role of surgical palliation. Am Surg. 2004; 70:433–437.
5. Benoist S, Pautrat K, Mitry E, Rougier P, Penna C, Nordlinger B. Treatment strategy for patients with colorectal cancer and synchronous irresectable liver metastases. Br J Surg. 2005; 92:1155–1160.

6. Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015; 33:1809–1824.

7. Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015; 14:1–10.

8. Scoggins CR, Meszoely IM, Blanke CD, Beauchamp RD, Leach SD. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol. 1999; 6:651–657.

9. Crane CH, Janjan NA, Abbruzzese JL, Curley S, Vauthey J, Sawaf HB, et al. Effective pelvic symptom control using initial chemoradiation without colostomy in metastatic rectal cancer. Int J Radiat Oncol Biol Phys. 2001; 49:107–116.

10. Tebbutt NC, Norman AR, Cunningham D, Hill ME, Tait D, Oates J, et al. Intestinal complications after chemotherapy for patients with unresected primary colorectal cancer and synchronous metastases. Gut. 2003; 52:568–573.

11. Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009; 27:3379–3384.

12. Clancy C, Burke JP, Barry M, Kalady MF, Calvin Coffey J. A meta-analysis to determine the effect of primary tumor resection for stage IV colorectal cancer with unresectable metastases on patient survival. Ann Surg Oncol. 2014; 21:3900–3908.

13. Lee KC, Ou YC, Hu WH, Liu CC, Chen HH. Meta-analysis of outcomes of patients with stage IV colorectal cancer managed with chemotherapy/radiochemotherapy with and without primary tumor resection. Onco Targets Ther. 2016; 9:7059–7069.

14. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009; 151:264–269.

15. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Oxford (UK): The Cochrane Collaboration;2011.
16. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa (ON): Ottawa Hospital Research Institute;c2018. cited 2017 Jun 27. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–560.

19. Thabane L, Akhtar-Danesh N. Guidelines for reporting descriptive statistics in health research. Nurse Res. 2008; 15:72–81.

20. Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008; 37:1148–1157.

21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.

22. Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on metaanalyses. BMJ. 2000; 320:1574–1577.

23. Tanoue Y, Tanaka N, Nomura Y. Primary site resection is superior for incurable metastatic colorectal cancer. World J Gastroenterol. 2010; 16:3561–3566.

24. Venderbosch S, de Wilt JH, Teerenstra S, Loosveld OJ, van Bochove A, Sinnige HA, et al. Prognostic value of resection of primary tumor in patients with stage IV colorectal cancer: retrospective analysis of two randomized studies and a review of the literature. Ann Surg Oncol. 2011; 18:3252–3260.

25. Verberne CJ, de Bock GH, Pijl ME, Baas PC, Siesling S, Wiggers T. Palliative resection of the primary tumour in stage IV rectal cancer. Colorectal Dis. 2012; 14:314–319.

26. Kim SK, Lee CH, Lee MR, Kim JH. Multivariate analysis of the survival rate for treatment modalities in incurable stage IV colorectal cancer. J Korean Soc Coloproctol. 2012; 28:35–41.

27. Park JH, Kim TY, Lee KH, Han SW, Oh DY, Im SA, et al. The beneficial effect of palliative resection in metastatic colorectal cancer. Br J Cancer. 2013; 108:1425–1431.

28. Boselli C, Renzi C, Gemini A, Castellani E, Trastulli S, Desiderio J, et al. Surgery in asymptomatic patients with colorectal cancer and unresectable liver metastases: the authors' experience. Onco Targets Ther. 2013; 6:267–272.
29. Yoon YS, Kim CW, Lim SB, Yu CS, Kim SY, Kim TW, et al. Palliative surgery in patients with unresectable colorectal liver metastases: a propensity score matching analysis. J Surg Oncol. 2014; 109:239–244.

30. Miyamoto Y, Watanabe M, Sakamoto Y, Shigaki H, Murata A, Sugihara H, et al. Evaluation of the necessity of primary tumor resection for synchronous metastatic colorectal cancer. Surg Today. 2014; 44:2287–2292.

31. Gresham G, Renouf DJ, Chan M, Kennecke HF, Lim HJ, Brown C, et al. Association between palliative resection of the primary tumor and overall survival in a population-based cohort of metastatic colorectal cancer patients. Ann Surg Oncol. 2014; 21:3917–3923.

32. de Mestier L, Neuzillet C, Pozet A, Desot E, Deguelte-Lardiere S, Volet J, et al. Is primary tumor resection associated with a longer survival in colon cancer and unresectable synchronous metastases? A 4-year multicentre experience. Eur J Surg Oncol. 2014; 40:685–691.

33. Kodaz H, Erdogan B, Hacibekiroglu I, Turkmen E, Tozkir H, Albayrak D, et al. Primary tumor resection offers higher survival advantage in KRAS mutant metastatic colorectal cancer patients. Hepatogastroenterology. 2015; 62:876–879.
34. Wong SF, Wong HL, Field KM, Kosmider S, Tie J, Wong R, et al. Primary tumor resection and overall survival in patients with metastatic colorectal cancer treated with palliative intent. Clin Colorectal Cancer. 2016; 15:e125–e132.

35. Wang Z, Liang L, Yu Y, Wang Y, Zhuang R, Chen Y, et al. Primary tumour resection could improve the survival of unresectable metastatic colorectal cancer patients receiving bevacizumab-containing chemotherapy. Cell Physiol Biochem. 2016; 39:1239–1246.

36. 't Lam-Boer J, Van der Geest LG, Verhoef C, Elferink ME, Koopman M, de Wilt JH. Palliative resection of the primary tumor is associated with improved overall survival in incurable stage IV colorectal cancer: a nationwide population-based propensity-score adjusted study in the Netherlands. Int J Cancer. 2016; 139:2082–2094.
37. Gulack BC, Nussbaum DP, Keenan JE, Ganapathi AM, Sun Z, Worni M, et al. Surgical resection of the primary tumor in stage IV colorectal cancer without metastasectomy is associated with improved overall survival compared with chemotherapy/radiation therapy alone. Dis Colon Rectum. 2016; 59:299–305.

38. Alawadi Z, Phatak UR, Hu CY, Bailey CE, You YN, Kao LS, et al. Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer. 2017; 123:1124–1133.

39. Ahmed S, Leis A, Chandra-Kanthan S, Fields A, Reeder B, Iqbal N, et al. Surgical management of the primary tumor in stage IV colorectal cancer: a confirmatory retrospective cohort study. J Cancer. 2016; 7:837–845.

40. Tsang WY, Ziogas A, Lin BS, Seery TE, Karnes W, Stamos MJ, et al. Role of primary tumor resection among chemotherapytreated patients with synchronous stage IV colorectal cancer: a survival analysis. J Gastrointest Surg. 2014; 18:592–598.

41. Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan Study Group. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med. 2000; 343:905–914.

42. Ghiringhelli F, Bichard D, Limat S, Lorgis V, Vincent J, Borg C, et al. Bevacizumab efficacy in metastatic colorectal cancer is dependent on primary tumor resection. Ann Surg Oncol. 2014; 21:1632–1640.

43. Cambien B, Richard-Fiardo P, Karimdjee BF, Martini V, Ferrua B, Pitard B, et al. CCL5 neutralization restricts cancer growth and potentiates the targeting of PDGFRβ in colorectal carcinoma. PLoS One. 2011; 6:e28842.

44. Chen HJ, Edwards R, Tucci S, Bu P, Milsom J, Lee S, et al. Chemokine 25-induced signaling suppresses colon cancer invasion and metastasis. J Clin Invest. 2012; 122:3184–3196.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download