Abstract
Estradiol (17β-estradiol) is synthesized primarily in the gonads of both sexes and regulates the development and function of reproductive organs. Recently, we reported that intestinal lymphocyte homeostasis is regulated by estradiol synthesized de novo in the endothelial cells of the high endothelial venules (HEVs) of mesenteric lymph nodes and Peyer's patches in mice. This observation prompted us to hypothesize that HEVs of intestinal lymphoid tissues are sites of estradiol synthesis across species. In this study, we examined whether estradiol is synthesized in the intestinal lymphoid tissues of adolescent piglets. Comparisons of estradiol levels in blood and tissue showed that estradiol concentrations in mesenteric lymph nodes and Peyer's patches were significantly higher than the level in serum. Reverse transcription polymerase chain reaction showed that porcine intestinal lymphoid tissues express mRNAs for steroidogenic enzymes (StAR, 17β-Hsd, 3β-Hsd, Cyp17a1, and Cyp19a1), and immunohistochemical results in ilial tissue showed expression of aromatase (CYP19) in Peyer's patch-localized endothelial cells of HEVs. When mesenteric lymph node and Peyer's patch tissues were cultured in vitro, they produced estradiol. Taken together, the results indicate that mesenteric lymph nodes and Peyer's patches are sites of estradiol synthesis in adolescent piglets.
Estradiol (17β-estradiol) is a reproductive hormone that is synthesized primarily by the gonads and regulates development and function of the reproductive organs. It also stimulates cell proliferation [22], has anti-inflammatory functions [14], promotes tissue survival [35], and enhances cardiovascular health [7], memory [15], and mood [32]. Estradiol deficiency leads to acne [18], and it is a contributing factor to ovulatory dysfunction [29], menopausal symptoms [31], insulin resistance [17], Alzheimer's disease [27], and bone loss [37]. Estradiol's novel roles in non-reproductive organs have been reported, and efforts have been made to search for the possibilities of estradiol synthesis in extra-gonadal sites. Thus far, bone [34], brain [6], liver [12], adipose tissue [5], skin [3], blood vessels [11], and spleen [33] have been identified as sites of estradiol synthesis; sites in which aromatase (P450arom; CYP19), a key enzyme in estradiol synthesis, is expressed [1].
In the digestive tract, estradiol regulates appetite [21], prevents colon cancer development [836], alleviates syndromes of inflammatory bowel diseases [23], decreases metabolic syndrome in menopausal women [4], and modulates gut microbiome and lymphocyte homeostasis [1019]. Recently, we reported that estradiol is synthesized in murine Peyer's patch (Pp) and mesenteric lymph node (mLN) tissues and has a role in regulating lymphocyte homeostasis in those secondary lymphoid tissues [24]. In this study, to determine whether estrogen synthesis in intestinal lymphoid tissues is conserved in other mammals, we examined the estrogen synthesis capacity of Pp and mLN tissues in pigs.
Minimal essential medium alpha (α-MEM) was purchased from Invitrogen (USA), fetal bovine serum (FBS) from American Type Culture Collection (ATCC; USA), insulin-transferrin-selenium (ITS) from VWR (USA), and androstenedione from Sigma (USA). The sensitive estradiol enzyme-linked immunosorbent assay (ELISA) kit was purchased from DRG International (USA).
This study was performed in accordance with recommendations in Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animal handling was done in accordance with the protocols approved by the University of Illinois at Urbana-Champaign's Institutional Animal Care and Use Committee (protocol No. 16036). Seven naturally farrowed domestic female Yorkshire piglets aged 3 weeks (n = 3) and 12 weeks (n = 4) were obtained from the University of Illinois swine herd and used in this study.
To measure estradiol-synthetic capacity, Pp, mLN, and ovary tissues were dissected from individual piglets euthanized by intracardiac administration of sodium pentobarbital (78 mg/kg body weight, Fatal Plus; Vortech Pharmaceuticals, USA), weighed, and cultured overnight in steroid-free media (α-MEM supplemented with 1% ITS [10 ng/mL insulin, 5.5 ng/mL transferrin, 5.5 ng/mL selenium], 5% FBS, and penicillin-streptomycin). Ovary tissue was then washed with fresh media to remove any steroid that might have been released to the culture media. Tissues were further cultured in the absence (vehicle) or presence of androstenedione (200 nM) [24]. Media were collected at 24, 48, and 72 h to measure estradiol content.
Estradiol concentrations were measured in blood collected via cardiac puncture, as well as in culture media and tissues (mLNs and gonads). Culture media estradiol content was measured directly, whereas, for blood and tissue samples, total lipids were extracted, following a standard procedure [25], and stored at −80℃ until needed for hormone assay. The concentrations of estradiol in the extracted lipids were measured by using ELISA (EIA-4399; DRG International, Germany) as previously reported [24]. Final estradiol concentrations were expressed by weight per unit volume or by weight of tissue, sera, or culture media (pg/mL or pg/mg). The samples were run in triplicate and had intra- and inter-assay coefficients of variability below 10%. The detection range was 0.1 to 30 pg/mL.
For gene expression analysis, mLNs, Pps, and ovaries were collected. Total RNA was extracted, by using TrizolVR solution (Ambion, USA), from porcine tissues (Pps, mLNs, and gonads) and then purified with an RNEasy Kit (Qiagen, USA). Concentration and quality of total RNA were analyzed by using a Nanodrop spectrophotometer (Thermo Scientific, USA) and the samples were then stored at −80℃ until use. Complementary DNA was generated by using M-MLV Reverse Transcriptase (Thermo Scientific) and random primers. The following primers were used for reverse transcription polymerase chain reaction (RT-PCR): 17β-Hsd 5′-AGC CAG AAT ATG TGG CAC CC-3′/5′-CAA CAA GTC CTG ATG GGG CT-3′, Cyp19a1 5′-GGA AAT CCA GAC TGT TGT TG-3′/5′-GCT GGA AGT ACC TGT AAG GA-3′, StAR 5′-GAC TTT GTC GGC TGT-3′/5′-ATC CCT TGA GGT CAA TGC TC-3′, 3β-Hsd 5′-CGT CCT GAC ACA CAA CTC CAA-3′/5′-CCA CGT TGC CGA CGT AGA-3′, CYP17a1 5′-TCC GAG AGG TGC TTC GAT TC-3′/5′-GGC GCT CCT TGA TCT TCA CT-3′, Rpl19 5′-CCT GAA GGT CAA AGG GAA TGT G-3′/5′-GTC TGC CTT CAG CTT GTG GAT-3′. PCR products were run on 2% agarose gels containing ethidium bromide and photographed under UV light. The RT-PCR products were quantified by determining band intensities measured with 1Dscan EX (Scanalytics, USA); Rpl19 was used as the internal control.
Collected tissues were fixed in 4% paraformaldehyde, embedded in paraffin wax, sectioned at 6 µm thickness, and stained with hematoxylin and eosin. Immunofluorescent labeling of CYP19 and PNAd were performed as previously described [24] by using monoclonal anti-CYP19 (MCA2077S; Bio-Rad Laboratories, USA) or purified rat anti-mouse PNAd carbohydrate epitope (MECA-79; BD Biosciences, USA). After primary antibody incubation overnight, slides were incubated with a secondary biotinylated horse anti-mouse IgG (Vectastain ABC kit; Vector Labs, USA) or rabbit anti-rat IgG (Vectastain ABC kit) and then treated with avidin-biotin complex solution (Vectastain Elite ABC kit; Vector Labs). For color development, 3,′-diaminobenzidine (DAB; Vector Labs) was applied. Slides were then counter-stained with hematoxylin, mounted, and imaged by using an Olympus BX51 microscope (Olympus, Japan).
Datasets were first tested for normality and homogeneity of variance and were transformed before statistical analysis. All figures depict non-transformed data. Statistical analyses were performed by applying two-tailed Student's t-test or one-way analysis of variance (ANOVA) followed by Tukey's honest significant difference.
In porcine species, the ileum contains multiple Pps. At three weeks of age, Pps are visibly identifiable by their unique luminal form, which appears as whitish round segments. These segments are follicles that contain densely populated lymphocytes (panel A in Fig. 1; insets). In pigs at this age, mLNs are easily identifiable as they are morphologically distinctive, but the follicles in this organ are less clearly defined than those in Pps (panel B in Fig. 1). The estradiol concentrations of serum, mLN, and ovary were 0.27 pg/mg, 0.66 pg/mg, and 1.84 pg/mg, respectively (panel C in Fig. 1). To see if lymphoid tissues synthesize estradiol, Pps-containing and Pp-free ileal tissue samples were cultured for 72 h, and the estradiol concentrations in the culture media were measured. During the culture period, estradiol concentrations continued to rise in the culture media containing Pps, but not in the media without Pps (panel D in Fig. 1). When the estradiol-synthetic capacity of the Pps was compared with those of mLN and ovary in 12-week-old pigs, the mLN and Pp samples exhibited a robust estradiol-synthetic capacity (Fig. 2). Notably, the estradiol-synthetic capacity of mLN (12.36 pg/mg at 72 h) was comparable to that of ovary (12.45 pg/mg at 72 h). Addition of androstenedione to the tissue cultures increased estradiol production slightly, but not significantly, in all examined tissues.
De novo estradiol synthesis in tissue requires the expression of StAR, 3β-Hsd, Cyp17a1, Cyp19a1, and 17β-Hsd genes (panel A in Fig. 3). To determine whether mLNs and Pps synthesize estradiol de novo, mRNA expression levels of steroidogenic genes were examined by performing RT-PCR (panel B in Fig. 3). Both Pps and mLNs expressed mRNAs for all of the steroidogenic genes examined, with minor differences in expression levels. Specifically, 3β-Hsd mRNA expression in both Pps and mLNs were significantly lower than the ovarian expression level (p < 0.01). The expression levels of 17β-Hsd in Pps and mLNs were 2-fold higher than that in ovary (p < 0.01). Pps and mLNs showed lower Cyp19a1 mRNA expression than that in ovary (panel B in Fig. 3). Importantly, immunohistochemical results showed that ileal tissue containing Pps of 12-week-old piglets expresses aromatase protein (CYP19) in endothelial cells of high endothelial venules (HEVs) (Fig. 4).
Both mLN and Pp tissues function at the forefront of immune surveillance in the gastrointestinal tract. They recruit, store, and dispatch lymphocytes, which serve as the first respondents to external signals that trigger immune responses [913]. This study aimed to determine whether Pps and mLNs of gilts synthesize estradiol.
If a tissue synthesizes estradiol, the estradiol concentration in that tissue will most likely be higher than the level in circulation. In the three-week-old piglets, the mLN and Pp tissues contained estradiol at higher concentrations than the serum level, indicating an estradiol-synthetic capacity of these two lymphoid tissues. We verified their estradiol-synthetic capacity by culturing them in vitro and measuring estradiol concentration in the tissue culture media. The tissue culture experiment revealed that ileal tissues containing Pps produced estradiol, but the Pp-free ileal tissue did not, indicating that the estradiol synthesis machinery resides within the Pp. This finding is consistent with a previous study showing that Pps, but not Pp-free ileal tissues, synthesize estradiol in mice [24].
Porcine gilts reach puberty as early as 12 weeks of age, at which point the steroidogenic activity of their ovaries begins, thereby producing a large amount of estradiol that triggers a preovulatory luteinizing hormone surge [226]. To compare the estradiol-synthetic capacity of Pps and mLNs with peri-pubertal ovaries, this study used 12-week-old piglets. Surprisingly, our results showed that mLN and ovary tissues had comparable estradiol-synthetic capacity.
Measurement of RNA transcription showed that Pps and mLNs express mRNAs for steroidogenic enzymes related to estradiol precursor production (StAR, 3β-Hsd, and Cyp17a1) and estradiol synthesis (Cyp19a1 and 17β-Hsd). This result indicates that Pps and mLNs may, similar to that in murine counterparts [24], synthesize estradiol de novo, starting from cholesterol (Fig. 3). In support of those results, both the mLN and Pp tissues synthesized estradiol without the addition of androstenedione to the culture media. Of note, 3β-Hsd mRNA expression levels in Pps and mLNs were significantly lower than that in ovarian tissues. Interestingly, 17β-Hsd and Cyp19a1 mRNA expression patterns in ovary were the opposite of the patterns observed in the lymphoid tissues: 17β-Hsd mRNA expression was higher in lymphoid tissues than in ovary, while Cyp19a1 mRNA expression was lower in lymphoid tissues than in ovary. These results suggest that, in Pp and mLN tissues, 17β-HSD may play a significant role in producing estradiol. In the immunohistochemical results, PNAd (a HEV marker) was observed to be localized with CYP19 in the endothelial cells of HEV, suggesting that these cells are the cell types responsible for estradiol synthesis in the examined lymphoid tissues. This result is not surprising because the same cell type was reported to be estrogen-producing in the murine gastrointestinal tract [24].
The roles that estradiol have in the examined lymphoid tissues are yet to be determined. However, estradiol probably has a direct effect on the function and behavior of lymphocytes (cytokine production, differentiation, and proliferation) [16202830]. A homeostatic role of locally synthesized estradiol has been suggested [24], with the implication that dysregulated estradiol synthesis in the gastrointestinal tract may leave the intestine prone to hyperinflammation. Future studies may investigate this possibility and other roles of locally produced estradiol.
Figures and Tables
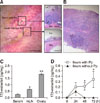 | Fig. 1Histological organization of Peyer's patch (Pp) and mesenteric lymph node (mLN) tissue, estradiol synthesis in dissected ileal Pp, and tissue estradiol concentrations. Tissues were dissected from a 3-week-old piglet. (A) Anatomical and histological view of representative Pp in luminal surface and of ileal tissues without Pp. Pp is indicated by arrows. Lu, lumen; Vi, villi; Mu, muscularis externa. (B) Histological view of mesenteric mLN with lymphoid follicle (LF) surrounded by the dotted line. (C) Concentrations of estradiol in serum, mLN, and ovary (n = 4, respectively). (D) Changes in estradiol concentration in ileal tissues with and without Pp. Tissues were dissected from 3-week old piglets (n = 4), cultured in vitro, and the amount of estradiol released from the tissues was measured until 72 h at 24 h intervals. Scale bars = 3 mm (A), 0.5 mm (insets in A), 0.3 mm (B). Significantly different from the ileum at *p < 0.01 or **p < 0.005, respectively, obtained via one-way ANOVA and Tukey's post hoc test. |
 | Fig. 2Estradiol synthesis in dissected ileal Peyer's patch (Pp), mesenteric lymph node (mLN), and ovary tissues showing changes in estradiol concentrations. Tissues were dissected from 12-week-old piglets and cultured in vitro (n = 4) in the absence (Veh) or presence of androstenedione (AN; 200 nM). The amount of estradiol released from the tissues was measured until 72 h at 24 h intervals. |
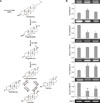 | Fig. 3Expression of steroidogenic enzyme genes in porcine Peyer's patch (Pp) and mesenteric lymph node (mLN) tissues in a 12-week-old pig. (A) Key enzymes responsible for precursor synthesis (StAR, 3β-HSD, and CYP17) and the rate-limiting estradiol-synthetic enzymes (17β-HSD and CYP19) in the steroidogenic pathway. (B) Quantitation of the relative mRNA expression and representative agarose gel images of StAR, 3β-Hsd, Cyp17a1, 17β-Hsd, and Cyp19a1 in ovary, ileal Pp, and mLN tissues of 12-week-old female piglets. Rpl19 was used for internal control. Significantly different from ovary at *p < 0.01 or **p < 0.005, respectively, based on one-way ANOVA and Tukey's post hoc test results (n = 4). Note that 17β-Hsd expression is higher in Pp and mLN than in ovary, whereas Cyp19a1 expression is lower in these lymphoid tissues than in the ovary. |
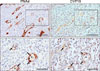 | Fig. 4Aromatase (CYP19) expression in high endothelial venule (HEV) cells of Peyer's patch (Pp) tissue. Adjacent sections of a Pp of a 12-week-old female piglet's ileum containing Pp were stained with antibodies against PNAd (a HEV marker) or CYP19. Lower panels show enlarged views of boxed areas in the upper panels. HEVs are indicated by asterisks. Arrows indicate CYP19-expressing HEV cells. Scale bars = 100 µm (upper), 50 µm (lower). |
References
1. Barakat R, Oakley O, Kim H, Jin J, Ko CJ. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016; 49:488–496.

2. Bazer FW, Thatcher WW, Martinat-Botte F, Terqui M. Sexual maturation and morphological development of the reproductive tract in Large White and prolific Chinese Meishan pigs. J Reprod Fertil. 1988; 83:723–728.

3. Berkovitz GD, Brown TR, Fujimoto M. Aromatase activity in human skin fibroblasts grown in cell culture. Steroids. 1987; 50:281–295.

4. Chen KL, Madak-Erdogan Z. Estrogen and microbiota crosstalk: should we pay attention? Trends Endocrinol Metab. 2016; 27:752–755.

5. Cleland WH, Mendelson CR, Simpson ER. Aromatase activity of membrane fractions of human adipose tissue stromal cells and adipocytes. Endocrinology. 1983; 113:2155–2160.

6. Colciago A, Celotti F, Pravettoni A, Mornati O, Martini L, Negri-Cesi P. Dimorphic expression of testosterone metabolizing enzymes in the hypothalamic area of developing rats. Brain Res Dev Brain Res. 2005; 155:107–116.

7. Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013; 19:197–209.

8. D'Errico I, Moschetta A. Nuclear receptors, intestinal architecture and colon cancer: an intriguing link. Cell Mol Life Sci. 2008; 65:1523–1543.
9. Eberl G, Lochner M. The development of intestinal lymphoid tissues at the interface of self and microbiota. Mucosal Immunol. 2009; 2:478–485.

10. Gomez A, Luckey D, Taneja V. The gut microbiome in autoimmunity: sex matters. Clin Immunol. 2015; 159:154–162.

11. Harada N, Sasano H, Murakami H, Ohkuma T, Nagura H, Takagi Y. Localized expression of aromatase in human vascular tissues. Circ Res. 1999; 84:1285–1291.

12. Hata S, Miki Y, Saito R, Ishida K, Watanabe M, Sasano H. Aromatase in human liver and its diseases. Cancer Med. 2013; 2:305–315.

13. Jung C, Hugot JP, Barreau F. Peyer's patches: the immune sensors of the intestine. Int J Inflam. 2010; 2010:823710.

14. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015; 294:63–69.

15. Lan YL, Zhao J, Li S. Update on the neuroprotective effect of estrogen receptor alpha against Alzheimer's disease. J Alzheimers Dis. 2015; 43:1137–1148.

16. Lélu K, Laffont S, Delpy L, Paulet PE, Périnat T, Tschanz SA, Pelletier L, Engelhardt B, Guéry JC. Estrogen receptor XMLLink_XYZ signaling in T lymphocytes is required for estradiol- mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol. 2011; 187:2386–2393.

17. Lin S, Ji W. Association between insulin resistance and estrogen in sexual precocity of obese children. Exp Ther Med. 2016; 12:2497–2500.

18. Lucky AW, Henderson TA, Olson WH, Robisch DM, Lebwohl M, Swinyer LJ. Effectiveness of norgestimate and ethinyl estradiol in treating moderate acne vulgaris. J Am Acad Dermatol. 1997; 37:746–754.

19. Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013; 339:1084–1088.

20. McMurray RW. Estrogen, prolactin, and autoimmunity: actions and interactions. Int Immunopharmacol. 2001; 1:995–1008.

21. Mela V, Vargas A, Meza C, Kachani M, Wagner EJ. Modulatory influences of estradiol and other anorexigenic hormones on metabotropic, Gi/o-coupled receptor function in the hypothalamic control of energy homeostasis. J Steroid Biochem Mol Biol. 2016; 160:15–26.

22. Moravek MB, Yin P, Ono M, Coon JS V, Dyson MT, Navarro A, Marsh EE, Chakravarti D, Kim JJ, Wei JJ, Bulun SE. Ovarian steroids, stem cells and uterine leiomyoma: therapeutic implications. Hum Reprod Update. 2015; 21:1–12.

23. Mulak A, Taché Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol. 2014; 20:2433–2448.

24. Oakley OR, Kim KJ, Lin PC, Barakat R, Cacioppo JA, Li Z, Whitaker A, Chung KC, Mei W, Ko C. Estradiol synthesis in gut-associated lymphoid tissue: leukocyte regulation by a sexually monomorphic system. Endocrinology. 2016; 157:4579–4587.

25. Orczyk GP, Behrman HR. Ovulation blockade by aspirin or indomethacin--in vivo evidence for a role of prostaglandin in gonadotrophin secretion. Prostaglandins. 1972; 1:3–20.

26. Oxender WD, Colenbrander B, van deWiel DF, Wensing CJ. Ovarian development in fetal and prepubertal pigs. Biol Reprod. 1979; 21:715–721.

27. Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol. 1994; 140:256–261.

28. Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005; 97:107–113.

29. Polan ML, Totora M, Caldwell BV, DeCherney AH, Haseltine FP, Kase N. Abnormal ovarian cycles as diagnosed by ultrasound and serum estradiol levels. Fertil Steril. 1982; 37:342–347.

30. Priyanka HP, Krishnan HC, Singh RV, Hima L, Thyagarajan S. Estrogen modulates in vitro T cell responses in a concentration- and receptor-dependent manner: effects on intracellular molecular targets and antioxidant enzymes. Mol Immunol. 2013; 56:328–339.

31. Richelson LS, Wahner HW, Melton LJ 3rd, Riggs BL. Relative contributions of aging and estrogen deficiency to postmenopausal bone loss. N Engl J Med. 1984; 311:1273–1275.

32. Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014; 345:771–776.

33. Samy TS, Knöferl MW, Zheng R, Schwacha MG, Bland KI, Chaudry IH. Divergent immune responses in male and female mice after trauma-hemorrhage: dimorphic alterations in T lymphocyte steroidogenic enzyme activities. Endocrinology. 2001; 142:3519–3529.

34. Sasano H, Uzuki M, Sawai T, Nagura H, Matsunaga G, Kashimoto O, Harada N. Aromatase in human bone tissue. J Bone Miner Res. 1997; 12:1416–1423.

35. Schreihofer DA, Ma Y. Estrogen receptors and ischemic neuroprotection: who, what, where, and when? Brain Res. 2013; 1514:107–122.

36. Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor XMLLink_XYZ in colonic epithelium. Proc Natl Acad Sci U S A. 2006; 103:2959–2964.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download