Abstract
Porcine reproductive and respiratory syndrome (PRRS) is recognized as one of the most important infectious diseases causing serious economic loss in the swine industry worldwide. Due to its increasing genetic diversity, a rapid and accurate diagnosis is critical for PRRS control. The immunochromatographic strip test (ICST) is a rapid and convenient type of immunoassay. In this study, an on-site immunochromatographic assay-based diagnostic method was developed for detection of PRRS virus (PRRSV)-specific antibodies. The method utilized colloidal gold nanoparticle-labeled dual-type nucleocapsid proteins encoded by open reading frame 7. We evaluated 991 field samples from pig farms and 66 serum samples from experimentally PRRSV-inoculated pigs. Based on true PRRSV-specific antibody-positive or -negative sera determined by immunofluorescence assay and IgM enzyme-linked immunosorbent assay (ELISA), the specificity and sensitivity of the ICST were 97.5% and 91.1%, respectively, similar to those of a commercial ELISA (IDEXX PRRS X3 Ab). More importantly, the ICST was completed within 15 min and could detect the PRRSV-specific antibody at an earlier stage of infection (3–7 days) than that of ELISA (7+ days). The results demonstrate that the developed ICST has great potential as an on-farm diagnostic method, providing excellent diagnostic performance in a quick and convenient manner.
Porcine reproductive and respiratory syndrome (PRRS) is an infectious swine disease that causes respiratory illness in pigs of all ages and reproductive failure including early farrowing, late-term abortions, and mummified or stillborn fetus in pregnant gilts and sows [123]. Tremendous economic losses caused by decreased productivity have made this disease one of the major concerns in the pig industry worldwide [11]. Since there are reports of increasing genetic and antigenic diversities in the PRRS virus (PRRSV) circulating in Korea [56141518], a rapid, accurate, and easily performed diagnostic assay for use in local pig farms nationwide is critical for surveillance of PRRSV infection and control of its spread. To date, various approaches for detecting PRRSV-specific antibodies have been developed and applied. The commonly used serological diagnostic methods comprise indirect immunofluorescent antibody (IFA) assay, immunoperoxidase monolayer assay (IMPA), and enzyme-linked immunosorbent assay (ELISA) [371024]. ELISA is known as the most reliable and commonly used serological diagnostic method [25]. Although it shows high degrees of sensitivity and specificity to detect PRRSV-specific antibodies in swine sera, well-trained personnel and a time-consuming multistep procedure prior to obtaining final results are needed for IFA, IMPA, and ELISA, not to mention their relatively high cost. Furthermore, the aforementioned diagnostic tests need to be performed in a laboratory with specialized and expensive equipment. In contrast, an adequate on-site test, with rapidity and convenience and without additional expenses for shipping and analysis, would provide greater efficiency in the early control of PRRS compared to the aforementioned laboratory methods. The immunochromatographic strip test (ICST) developed in the 1980s [230] has been used to monitor various animal diseases [1319212729] owing to its several advantages, including simple and easy procedure, quick operation, and low cost [26]. In addition to excellent diagnostic efficacy, the capability of an ICST to detect antibodies at an earlier stage of infection than current diagnostic methods makes it highly useful for minimizing the economic impact of a disease outbreak. Despite the availability of commercial ICST for PRRSV-specific antibody detection, diagnostic performance of commercially available ICSTs in the field has not been well described. The aim of this study was to develop a rapid and sensitive ICST based on type 1 and type 2 N proteins and labeled with colloidal gold nanoparticles to be used for the detection of PRRSV-specific antibodies during the early period of PRRSV infection and to compare its diagnostic performance with a commercial ELISA against the valid reference standard IFA.
Two prototype PRRSVs (VR2332 and Lelystad virus [LV]), type 1 (E38) and type 2 (PL97-1 and LMY) field strains isolated from PRRS-affected swine farms in Korea were propagated in Marc-145 cells and stored in our laboratory at −70℃ until use. The nucleotide sequence identity of the ORF7 gene is 61.2% between VR2332 and LV, while 93.5% and 95.9% to 99.5% within type 1 and type 2 viruses used in this study, respectively. To evaluate the diagnostic performance (sensitivity and specificity) of the ICST, 991 sera samples from growing pigs (between 2 and 6 months old) were submitted to a diagnostic lab (Animal and Plant Quarantine Agency, Korea) from 71 domestic pig farms on which there were poor growth and respiratory illness between 2013 and 2014. The pig farms were located in provinces nationwide: Gyeonggi (n = 27), Chungbuk (n = 9), Chungnam (n = 10), Jeonbuk (n = 18), and Gyeongnam (n = 7). Collected samples were stored at −20℃ until use; at which time they were thawed and subjected to ELISA, IFA, and ICST.
To evaluate ICST in several PRRSV-positive serum samples and elucidate the temporal profiles of PRRSV-specific antibodies, six weaned pigs (3 weeks old) were purchased from a pig farm known to be free of PRRSV. The pigs were isolated for 7 days and then randomly assigned to three groups of two pigs each. Sera were collected from all pigs before challenge and were used as the known negative controls. The three groups of two pigs each were intramuscularly inoculated with 2 mL of one of three PRRSV field-isolated strains (PL97-1, E38, or LMY) at titers of 105 TCID50/mL (TCID50, 50% tissue culture infective dose). Sera of the challenged pigs were collected at 7, 14, 28, 39, and 52 days post-infection (dpi) and were used as the known positive samples. Additionally, to identify how early in the post-infection period these tests can detect PRRSV-specific antibodies following initial exposure to PRRSV, six weaned pigs (3 weeks old) of the same litter were randomly assigned to two groups of three pigs each. Two of those groups were intramuscularly inoculated with 2 mL of a PRRSV prototype virus (VR2332 or LV) at a titer of at least 105 TCID50/mL. Sera of the challenged pigs were collected at 0, 1, 3, 5, 7, and 14 dpi. Collected samples underwent IFA, ICST, and commercially available ELISA, and the results were compared. The experimental protocols for the care and use of laboratory animals were approved (approval No. 2017-277) by the Institutional Animal Care and Use Committee at the Animal and Plant Quarantine Agency, Korea.
Briefly, MARC-145 cells were seeded and grown in 96-well plates on RPMI 1640 growth medium (Invitrogen, USA) containing 10% fetal bovine serum (Gibco-BRL, USA) and 1× antibiotics (Gibco-BRL) for 16 h, and inoculated with VR2332 or LV strains at a titer of 105 TCID50/mL. The infected plates were incubated for 24 h and, after removing the media, fixed in 80% cold acetone in methanol for 10 min at −20℃. Inactivated sera were diluted 1:20 in phosphate-buffered saline (PBS) and transferred to inoculated plates. After 30 min of incubation, the plates were washed with PBS. Then, fluorescein isothiocyanate-labeled rabbit anti-swine IgG was added to each well at a dilution of 1:200. Following incubation for 1 h, the plates were PBS-washed and examined under a fluorescence microscope (Olympus, Japan).
To determine the relative efficacy of ICST, commercial indirect IgG ELISA (IDEXX PRRS X3 Ab; IDEXX Laboratories, USA) and an in-house IgM ELISA. Assays were performed following the appropriate set of instructions. The optical density (OD) of each well was measured at 650 nm with a spectrophotometer (Sunrise Microplate Reader, Switzerland) and the results converted into sample-to-positive (S/P) ratios. Samples with an S/P ratio equal to or greater than 0.4 were considered to be positive for IgG antibody against PRRSV.
To assess the PRRSV-specific IgM antibody response, the commercial indirect IgG ELISA (IDEXX PRRS X3 Ab; IDEXX Laboratories) was performed in an identical manner to that described above with one exception. HRP-labeled goat anti-swine IgM antibody (30 ng/mL) was used instead of the anti-swine IgG antibody conjugate provided in the IDEXX kit. As ODs of all serum samples obtained from the PRRS-negative farms were less than 0.1, samples with OD higher than 0.1 were considered to be positive for the IgM antibody against PRRSV.
To obtain recombinant nucleocapsid (rN) protein, the RNAs from the VR2332 and LV strains were extracted by using the RNeasy Mini Kit (Qiagen, USA). The N protein-coding gene was amplified by using the Power cDNA Synthesis Kit (iNtRON Biotechnology, Korea) and the Taq PCR Master Mix Kit (Qiagen) with the following primers: N gene of VR2332 forward primer, 5′-GGATCCATGCCAAATAACAACGGCAAGCAG-3′; N gene of VR2332 reverse primer, 5′-AAGCTTTCATGCTGAGGGTGATGCTGTGAC-3′. N gene of LV forward primer, 5′-GGATCCATGGCCGGTAAAAACCAGAGCCAG-3′; N gene of LV type reverse primer, 5′-AAGCTTACTTGCACCCTGACTGGCGGA-3′. Each amplicon containing the BamH1/HindIII restriction enzyme site was cloned into the pET32a vector and expressed to 6× His-tagged rN proteins (VR2332 or LV) in the Escherichia coli host strain BL21 (DE3). After induction of 1 mM of isopropyl–β-D-thiogalactopyranoside (IPTG) for 24 h at 20℃, the bacterial cell pellet was resuspended and lysed by sonication in lysis buffer (20 mM imidazole in PBS, pH 7.4) and then centrifuged at 800 × g for 20 min at 4℃. The supernatants were introduced to the HisTrap HP (GE Healthcare, UK), and 6× His-tagged rN proteins were eluted by elution buffer (500 mM imidazole in PBS, pH 7.4) after washing with washing buffer (60 mM imidazole in PBS, pH 7.4). The purified rNs were evaluated by SDS-PAGE analysis for purity (data not shown).
To prepare the colloidal gold PRRSV antigen conjugate, one milliliter of 1% solution of HAuCl4 was added to a 100 mL of double-distilled water and boiled. Next, 2.4 mL of a 1% solution of trisodium citrate dihydrate was added to the boiling solution. The gold solution was gradually cooled and adjusted to pH 9.0 by adding 0.1 M of potassium carbonate solution appropriately. The rN protein (5 µg/mL) was added to 30 mL of the gold nanoparticle solution for gold conjugation. After 30 min of incubation at room temperature, 0.3% casein was added to the conjugate solution for blocking. The solution was additionally incubated for 30 min at room temperature and then centrifuged at 12,000 × g for 20 min. The supernatant was removed and the gold conjugate resuspended in 3 mL of conjugate washing buffer (1% BSA in 2 mM borax, pH 9.0), and the final OD was measured by spectrophotometer at 525 nm absorbance.
The developed immunochromatographic test strip is composed sample and gold conjugate pads, a nitrocellulose membrane, and an absorption pad on a plastic backing card (PJ Company, Korea) as illustrated in Fig. 1. The rN proteins of type 1 and type 2 PRRSV were immobilized on the nitrocellulose membrane (Millipore, USA) as capture antigens, and the gold conjugate was prepared by conjugation between 40 nm diameter colloidal gold and the mixture of PRRSV rN proteins. The 0.8 mg/mL of rN protein mixture (from type 1 and type 2 PRRSV) and 2 mg/mL of anti-rabbit IgG were immobilized as test and control lines, respectively, on the nitrocellulose membrane. The gold nanoparticle-conjugated rN proteins (OD525 nm = 7.0) and the gold nanoparticle-conjugated rabbit IgG (OD525 nm = 3.0) were air-dried in a glass fiber sheet with a stabilizer to form the gold conjugate pad. Assembled strips are stable for 1 year under the recommended storage condition (4℃–30℃). Swine serum (20 µL) was added to 80 µL of sample dilution buffer (Tween-20 0.4% in 50 mM of sodium tetraborate-12H2O, pH 9.0), and the mixture was added to the sample loading well in the test strip. After 10 min, the test result can be read by using a time-resolved fluorescence (TRF) reader (Medisensor, Korea) or naked eyes. Test-line intensity is able to be expressed as a numerical value by the TRF reader in accord with the concentration of the antibodies against the PRRSV in swine serum. A TRF detector value equal to or greater than 40 was determined to be positive. Although there was no difference between a TRF reader and naked eyes in reading the results, the TRF reader was used to compare the slight difference in numerical values with the ELISA results. As shown in Fig. 2, if swine serum contains specific antibodies against type 1 and/or type 2 PRRSV, the antibodies will bind to the rN protein-conjugated colloidal gold, and this primary immune complex moves through the nitrocellulose membrane due to capillary force. The secondary immune complex (rN protein–anti-PRRSV antibody–rN protein-conjugated colloidal gold) is formed in the test line of the nitrocellulose membrane. If swine serum contains no antibodies against the PRRSV, the primary or secondary immune complexes will not be formed in the test line, thus only the control line will be generated.
To compare diagnostic specificity and sensitivity of the developed ICST with ELISA, 991 field samples were processed with both the IDEXX ELISA and the developed ICST. In addition, IFA was performed with the same serum samples as a reference method. As shown in Table 1, the IFA results of the 991 field samples indicated that there were 583 positive and 408 negative samples. The ICST PRRSV results showed 97.4% sensitivity and 88.2% specificity, whereas the commercial ELISA showed 97.8% sensitivity and 92.4% specificity (Table 1). A few ambiguous samples that yielded unmatched results among ELISA, ICST, and IFA were retested with IgM ELISA. The efficacy of ICST and commercial ELISA determined by IFA and by IgM ELISA as reference methods is also summarized in Table 1. The ICST for PRRSV showed 97.5% sensitivity and 91.1% specificity, whereas the commercial ELISA showed 97.3% sensitivity and 94.7% specificity (Table 1).
Results of 36 serum samples from 6 experimentally infected pigs are depicted in Fig. 3. The PRRSV-specific antibody response of the ICST was compared with that of the commercial ELISA. All pigs were seronegative to PRRSV at 0 dpi. By using the IDEXX X3 Ab ELISA, 4 of 6 infected pigs seroconverted at 7 dpi and all were clearly positive at 14 dpi. On the other hand, the developed ICST was slightly more sensitive than ELISA. Using the ICST, seroconversion was observed in all 6 pigs from day 7 dpi onwards, except one pig. In addition, there were some discrepancies in trends of detection of PRRSV-specific antibodies between ICST and commercial ELISA. Briefly, the ICST results showed a peak at 7 dpi, being followed by a steep decrease and a gradual increase, in 5 of the 6 pigs. Therefore, we performed in-house IgM ELISA with the same sera from 3 of 6 pigs (one pig in each group) to identify whether the developed ICST can detect another isotype of PRRSV-specific antibodies and to determine what may cause the sharp increases in PRRSV-specific antibody response at 7 dpi. The result revealed that a similar pattern existed in the appearance of PRRSV- specific antibodies between the ICST and the in-house IgM ELISA, particularly in the early infection period (Fig. 4).
To identify how early in the early post-infection period following initial exposure to PRRSV the ICST PRRSV-specific antibodies can be detected, six weaned pigs (3 weeks old) of the same litter were randomly assigned to two groups of three pigs each. Two of the groups of three pigs each were intramuscularly inoculated with 2 mL of PRRSV prototype viruses VR2332 or LV at a titer of 105 TCID50/mL. The sera of the challenged pigs were collected at 0, 1, 3, 5, 7, and 14 dpi. Using an S/P ratio of 0.4 as the cutoff, only 1 of 6 pigs had antibody detectable by commercial ELISA at 7 dpi, whereas the ICST detected antibodies in 1 of 6 pigs at 3 dpi and in 6 of 6 pigs at 7 dpi (Fig. 5). All 6 pigs were clearly positive for PRRSV-specific antibodies at 14 days post challenge with both ELISA and ICST (data not shown).
Several studies have described increasing incidences of PRRS caused by single or concurrent infection of type 1 and type 2 PRRS field viruses that show an expanding genetic diversity in Korea [56141518]. Simplicity and accuracy of PRRS diagnosis are highly important for the creation of prompt strategies for minimizing PRRS affliction. In accordance with those needs, the ICST reported in this study was developed to have diagnostic features and to be applicable for use in the field.
The ICSTs previously developed for PRRSV antibody detection selected the N and/or M protein of a single PRRSV strain to capture PRRSV-specific antibodies in a test sample [81933] due to the strong and broad antigenicity of those proteins [922]. Those ICSTs utilized colloidal gold conjugated with protein G and/or A. The PRRSV-specific-antibody detection capabilities of those ICSTs were directly compared with those of two commercial ELISAs (HerdChekt PRRS ELISA kit and CIVTEST SUIS PRRS ELISA kit). Meanwhile, the ICST developed in the current study used a mixture of rN proteins from both type 1 and type 2 PRRSVs as capture antigens by being labeled with colloidal gold nanoparticles, which was applied to test line. The use of dual-type N proteins was expected to enhance diagnostic performance due to the genetic variation between the two PRRSV types and to improve the diagnostic performance in ELISA by utilizing N proteins of both types when compared to that of single type [7].
The diagnostic performance of the developed ICST was determined by comparing its diagnostic efficacy (using IFA assay and IgM ELISA as valid standards) with that of a commercial ELISA (IDEXX PRRS X3 Ab) in 991 clinical serum samples and 66 serum samples from 12 experimentally inoculated pigs. The ICST in this study was shown to have similar levels of sensitivity (97.5% vs. 97.3%) and specificity (91.1% vs. 94.7%) to that of the IDEXX commercial ELISA and proved capable of detecting PRRSV-specific antibodies in pigs experimentally infected with three field (LMY, E38, and PL97-1) and two prototype viruses (VR2332 and LV).
Some features differentiate this newly developed ICST from previous ICSTs, and they provide several advantages. Firstly, our ICST used N proteins of dual-type (type 1 and type 2) PRRSVs. Currently, approximately 50% of PRRS-affected swine farms in Korea are infected by type 1 PRRSVs, while the others are infected by type 2 PRRSVs (data not shown). Typical of the PRRSV, fast antigenic mutation as well as substantial antigenic differences between two genotypes can often limit the sensitivity of the ICST when testing its validity in field samples. Therefore, application of N proteins of both virus genotypes was expected to enhance the sensitivity of our ICST. As shown in Figs. 3 and 5, it was shown that our ICST was able to detect PRRSV-specific antibodies from sera of pigs infected with type 1 (LV, E38) and type 2 (VR2332, PL97-1, LMY) PRRS prototype and field viruses. Secondly, our ICST was designed to detect various isotypes of PRRSV-specific antibodies such as IgG, IgM, and IgA by applying colloidal gold nanoparticle-conjugated rN proteins and rabbit IgG gold conjugates in a conjugate pad. The effectiveness of that strategy was shown in experimentally PRRSV-inoculated pigs. As depicted in Fig. 3, the detection dynamics of PRRSV-specific antibodies by ICST have been well matched with that of commercial ELISA, except that the antibody level estimated by ICST reached a peak at 7 dpi, which is different from observations that PRRSV-specific antibodies increase gradually and reach a peak after 2 to 3 weeks [1628]. Thus, we additionally carried out an in-house IgM ELISA to confirm whether our ICST can detect the IgM antibody in the early infection period. Based on extensive study of antibody isotype profiles, IgM is the immunoglobulin which appears first when a virus infection occurs and disappears rapidly after the IgG antibody appears [420]. In a primary infection of PRRSV, IgG is first detected at 7 to 10 dpi and reaches a peak at 2 to 4 weeks post-infection [203132], whereas IgM is first detected in serum at 5 dpi and is detectable for up to 28 dpi when using the IFA assay [12] and is detected between 7 dpi and 20 dpi when using IMPA [17]. In this study, the ICST could detect a PRRSV-specific antibody response (possibly an IgM response) as early as 3 dpi. These results suggested that our ICST may efficiently identify either IgG or IgM antibody-positive pigs in the early stage of infection. Moreover, there is a good expectation of using our ICST for detection of PRRSV-specific IgA in saliva, feces, etc. In contrast, previous ICSTs [81933] using protein A/G conjugated with colloidal gold were mostly available only for IgG in serum, since protein A/G has little or weak affinity to isotypes other than IgG. We also evaluated cross-reactivity of the ICST with 59 sera that were tested positive for classical swine fever virus (n = 9), bovine viral diarrhea virus (n = 2), foot and mouth disease virus (n = 2), porcine circovirus type 2 (n = 10), Actinobacillus pluoropneumoniae type 2 (n = 9), A. pluoropneumoniae type 5 (n = 9), Hemophilus parasuis (n = 4), Mycoplasma hyopneumoniae (n = 4) and Pasturella multocida type A (n = 10) with a commercial ELISA (MEDIAN Diagnostics, Korea). The developed ICST showed clear negative signals with only a red band at the control line in all tested sera (data not shown), which indicated that detection of PRRSV-specific antibodies using the ICST developed in this study would not be interrupted by infection of other pathogens present in a pig farm.
In summary, the ICST developed in this study is suitable for on-farm use, and the strip test results are generated within approximately 15 min with only 20 µL of swine serum. The developed strip test shows similar performance to that of a commercial ELISA kit and makes it possible to detect PRRSV-specific antibody at an earlier stage of infection than that from commercial ELISA. As a simple, cost-efficient, sensitive, and specific test for application in screening of swine sera against PRRSV, we strongly suggest that the ICST described in this study could, in the near future, successfully replace the current use of commercial ELISA for on-farm use.
Figures and Tables
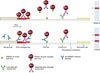 | Fig. 1Schematic diagram of immunochromatographic strip test (ICST) developed for porcine reproductive and respiratory syndrome virus (PRRSV)-specific antibodies. The strip is composed of sample pad, gold conjugate pad, nitrocellulose membrane, and absorption pad. If the serum contains PRRSV-specific antibodies, those antibodies are captured by recombinant nucleocapsid (rN) gold conjugates in the gold conjugate pad. The rN gold conjugate-antibody complex is then captured by the rN protein on the test line (T), producing a red visible band, while unbound rabbit IgG gold conjugates move through the nitrocellulose membrane and are captured by anti-rabbit IgG on the control line (C) to form another visible band. |
 | Fig. 2Examples of the developed immunochromatographic strip test results. (A) A positive result for diluted porcine reproductive and respiratory syndrome virus (PRRSV)-positive serum from experimentally PRRSV-infected pigs. (B) A negative result for diluted PRRSV-negative control serum from a 3-month-old pig purchased from a PRRSV-negative farm. C, control line; T, test line. |
 | Fig. 3Detection of porcine reproductive and respiratory syndrome virus (PRRSV)-specific antibodies in swine sera from 6 experimentally inoculated pigs by the developed immunochromatographic strip test (ICST) and commercial enzyme-linked immunosorbent assay (ELISA) (IDEXX PRRS X3 Ab; IDEXX Laboratories, USA). Six pigs in three groups (two pigs in each group) were inoculated with three types of PRRSVs including LMY, PL97-1, and E38, and sera were obtained at 0, 7, 14, 28, 39, and 52 days post-infection (dpi). The ICST detected PRRSV-specific antibodies in all 6 pigs as early as 7 dpi, while the commercial ELISA detected PRRSV-specific antibody in 4 of 6 pigs at 7 dpi. Samples with time-resolved fluorescence reader test intensities ≥ 40 were considered positive; samples with sample-to-positive (S/P) ratios ≥ 0.4 were considered positive. |
 | Fig. 4Porcine reproductive and respiratory syndrome virus (PRRSV)-specific antibody responses in immunochromatographic strip test (ICST) and IgM enzyme-linked immunosorbent assay (ELISA) (in-house) for swine sera from 3 experimentally inoculated pigs. The serum samples of pigs 36, 37, and 39 (one pig in each group) were applied to the in-house IgM ELISA and the results compared with the ICST results. The PRRSV-specific antibody profile showed a marked peak at 7 days post-infection (dpi). There were similar trends in antibody profiles of both ICST and IgM ELISA during the early infection period. OD, optical density. |
 | Fig. 5Detection of porcine reproductive and respiratory syndrome virus (PRRSV)-specific antibodies at different times after infection. Six pigs in two groups (three pigs each) were inoculated with two prototype PRRSVs (VR2332 and Lelystad virus [LV]) and sera were collected at 0, 1, 3, 5, 7, and 14 days post-infection (dpi). The immunochromatographic strip test (ICST) could detect PRRSV-specific antibody in 1 of 6 pigs at 3 dpi, 2 of 6 pigs at 5 dpi, and all pigs at 7 dpi, while the commercial enzyme-linked immunosorbent assay (ELISA) detected PRRSV-specific antibody in only 1 of 6 pigs (Pig 3) at 7 dpi. All pigs were positive for PRRSV-specific antibodies in both ELISA and ICST results from 14 dpi onwards (data not shown). Samples with time-resolved fluorescence reader test intensities ≥ 40 were considered positive; samples with sample-to-positive (S/P) ratios ≥ 0.4 were considered positive. |
Acknowledgments
This paper was written as part of a grant (code No. QIA I-1543083-2015-16-01) from Animal and Plant Quarantine Agency under the Ministry of Agriculture, Food, and Rural Affairs, Republic of Korea.
References
1. Albina E. Epidemiology of porcine reproductive and respiratory syndrome (PRRS): an overview. Vet Microbiol. 1997; 55:309–316.

2. Beggs M, Novotny M, Sampedro S, Devore J, Gordon J, Osikowicz G. A self performing chromatographic immunoassay for the qualitative determination of human chorionic gonadotrophin (HCG) in urine and sera. Clin Chem. 1990; 36:1084–1085.
3. Brown E, Lawson S, Welbon C, Gnanandarajah J, Li J, Murtaugh MP, Nelson EA, Molina RM, Zimmerman JJ, Rowland RR, Fang Y. Antibody response to porcine reproductive and respiratory syndrome virus (PRRSV) nonstructural proteins and implications for diagnostic detection and differentiation of PRRSV types I and II. Clin Vaccine Immunol. 2009; 16:628–635.

4. Carayannopoulos L, Capra ID. Immunoglobulins: structure and function. In : Paul WE, editor. Fundamental Immunology. 3rd ed. Raven Press: New York;1993. p. 283–314.
5. Cha SH, Choi EJ, Park JH, Yoon SR, Song JY, Kwon JH, Song HJ, Yoon KJ. Molecular characterization of recent Korean porcine reproductive and respiratory syndrome (PRRS) viruses and comparison to other Asian PRRS viruses. Vet Microbiol. 2006; 117:248–257.

6. Choi EJ, Lee CH, Song JY, Song HJ, Park CK, Kim B, Shin YK. Genetic diversity of porcine reproductive and respiratory syndrome virus in Korea. J Vet Sci. 2013; 14:115–124.

7. Chu JQ, Hu XM, Kim MC, Park CS, Jun MH. Development and validation of a recombinant nucleocapsid protein-based ELISA for detection of the antibody to porcine reproductive and respiratory syndrome virus. J Microbiol. 2009; 47:582–588.

8. Cui S, Zhou S, Chen C, Qi T, Zhang C, Oh J. A simple and rapid immunochromatographic strip test for detecting antibody to porcine reproductive and respiratory syndrome virus. J Virol Methods. 2008; 152:38–42.

9. Dea S, Gagnon CA, Mardassi H, Pirzadeh B, Rogan D. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) virus: comparison of the North American and European isolates. Arch Virol. 2000; 145:659–688.

10. Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Immune responses of pigs after experimental infection with a European strain of porcine reproductive and respiratory syndrome virus. J Gen Virol. 2005; 86:1943–1951.

11. Holtkamp DJ, Kliebenstein JB, Neumann E, Zimmerman JJ, Rotto HF, Yoder TK, Wang C, Yeske PE, Mowrer CL, Haley CA. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Produc. 2013; 21:72–84.
12. Joo HS, Park BK, Dee SA, Pijoan C. Indirect fluorescent IgM antibody response of pigs infected with porcine reproductive and respiratory syndrome syndrome virus. Vet Microbiol. 1997; 55:303–307.

13. Kameyama K, Sakoda Y, Tamai K, Igarashi H, Tajima M, Mochizuki T, Namba Y, Kida H. Development of an immunochromatographic test kit for rapid detection of bovine viral diarrhea virus antigen. J Virol Methods. 2006; 138:140–146.

14. Kim JY, Lee SY, Sur JH, Lyoo YS. Serological and genetic characterization of the European strain of the porcine reproductive and respiratory syndrome virus isolated in Korea. Korean J Vet Res. 2006; 46:363–370.
15. Kim SH, Roh IS, Choi EJ, Lee C, Lee CH, Lee KH, Lee KK, Song YK, Lee OS, Park CK. A molecular analysis of European porcine reproductive and respiratory syndrome virus isolated in South Korea. Vet Microbiol. 2010; 143:394–400.

16. Kittawornrat A, Engle M, Panyasing Y, Olsen C, Schwartz K, Rice A, Lizano S, Wang C, Zimmerman J. Kinetics of the porcine reproductive and respiratory syndrome virus (PRRSV) humoral immune response in swine serum and oral fluids collected from individual boars. BMC Vet Res. 2013; 9:61.

17. Labarque GG, Nauwynck HJ, Van Reeth K, Pensaert MB. Effect of cellular changes and onset of humoral immunity on the replication of porcine reproductive and respiratory syndrome virus in the lungs of pigs. J Gen Virol. 2000; 81:1327–1334.

18. Lee JA, Lee NH, Lee JB, Park SY, Song CS, Choi IS, Lee SW. Genetic diversity of the Korean field strains of porcine reproductive and respiratory syndrome virus. Infect Genet Evol. 2016; 40:288–294.

19. Li Y, Hou L, Ye J, Liu X, Dan H, Jin M, Chen H, Cao S. Development of a convenient immunochromatographic strip for the diagnosis of infection with Japanese encephalitis virus in swine. J Virol Methods. 2010; 168:51–56.

20. Loemba HD, Mounir S, Mardassi H, Archambault D, Dea S. Kinetics of humoral immune response to the major structural proteins of the porcine reproductive and respiratory syndrome virus. Arch Virol. 1996; 141:751–761.

21. Magar R, Larochelle R, Robinson Y, Dubuc C. Immunohistochemical detection of porcine reproductive and respiratory syndrome virus using colloidal gold. Can J Vet Res. 1993; 57:300–304.
23. Mengeling WL, Lager KM, Vorwald AC. Clinical effects of porcine reproductive and respiratory syndrome virus on pigs during the early postnatal interval. Am J Vet Res. 1998; 59:52–55.
24. Nodelijk G, Wensvoort G, Kroese B, van Leengoed L, Colijn E, Verheijden J. Comparison of a commercial ELISA and an immunoperoxidase monolayer assay to detect antibodies directed against porcine respiratory and reproductive syndrome virus. Vet Microbiol. 1996; 49:285–295.

25. Okinaga T, Yamagishi T, Yoshii M, Suzuki T, Miyazaki A, Takagi M, Tsunemitsu H. Evaluation of unexpected positive results from a commercial ELISA for antibodies to PRRSV. Vet Rec. 2009; 164:455–459.

26. Peng D, Hu S, Hua Y, Xiao Y, Li Z, Wang X, Bi D. Comparison of a new gold-immunochromatographic assay for the detection of antibodies against avian influenza virus with hemagglutination inhibition and agar gel immunodiffusion assays. Vet Immunol Immunopathol. 2007; 117:17–25.

27. Peng F, Wang Z, Zhang S, Wu R, Hu S, Li Z, Wang X, Bi D. Development of an immunochromatographic strip for rapid detection of H9 subtype avian influenza viruses. Clin Vaccine Immunol. 2008; 15:569–574.

28. Sattler T, Wodak E, Revilla-Fernández S, Schmoll F. Comparison of different commercial ELISAs for detection of antibodies against porcine respiratory and reproductive syndrome virus in serum. BMC Vet Res. 2014; 10:300.

29. Sithigorngul W, Rukpratanporn S, Sittidilokratna N, Pecharaburanin N, Longyant S, Chaivisuthangkura P, Sithigorngul P. A convenient immunochromatographic test strip for rapid diagnosis of yellow head virus infection in shrimp. J Virol Methods. 2007; 140:193–199.

30. Tanaka R, Yuhi T, Nagatani N, Endo T, Kerman K, Takamura Y, Tamiya E. A novel enhancement assay for immunochromatographic test strips using gold nanoparticles. Anal Bioanal Chem. 2006; 385:1414–1420.

31. Vézina SA, Loemba H, Fournier M, Dea S, Archambault D. Antibody production and blastogenic response in pigs experimentally infected with porcine reproductive and respiratory syndrome virus. Can J Vet Res. 1996; 60:94–99.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download