Abstract
We studied the toxic effects of a Sarcocystis hirsuta cyst extract fed to mice. Degenerative changes were found in mice gavage-fed fresh, frozen, and heat-treated S. hirsuta cyst extract. There were increases in the levels of serum aspartate aminotransferase and alanine aminotransferase as well as hepatic and brain malondialdehyde (MDA) levels along with concomitant decreases in catalase (CAT) and superoxide dismutase (SOD) activities of mice receiving fresh and frozen S. hirsuta extracts. Gavage feeding of heat-treated S. hirsuta cyst extract had no effects on liver enzymes or brain MDA content, but the liver MDA level did increase. Mice in the heat-treated cyst group showed reduced CAT and SOD activities as well as increased hepatic MDA levels compared to those in the control group. These results indicate that an extract of S. hirsuta cyst can induce oxidative stress and hepatic injury, even after heat treatment.
Sarcocystis is a parasitic protozoan, and most Sarcocystis species infect mammals, while others infect reptiles and birds. Sarcocystis hirsuta is a species that can infect cattle and produce macroscopic cysts, and it has been reported that most of the Sarcocystis cysts are not removed from carcasses during slaughterhouse processing [6]. Many studies have indicated a high prevalence of sarcocystosis in slaughtered bovines in Iran [15]. Cattle imported from India are being slaughtered in industrial centers in southeast Iran, and those cattle are reported to be highly infected with Sarcocystis spp., including S. hirsuta [19]. During the meat inspection process, not all Sarcocystis cysts are eliminated from carcasses; therefore, it is possible for consumers to ingest Sarcocystis cysts when eating raw, frozen, or cooked meat products.
The wall of a Sarcocystis cyst contains sarcocystin [4], a protein toxin, and, like other proteins, its biological activities can be affected by stomach enzymes and acid. Although toxins can be inactivated in the stomach, there are reports of acute poisoning following consumption of Sarcocystis-infected meat [2]. Various toxic effects of Sarcocystis cyst extracts have been investigated in laboratory animals following intraperitoneal or intravenous injections [113]. However, until now, there have been no reports on the potential toxic effects of a Sarcocystis cyst extract following oral administration in laboratory animals. Therefore, we undertook this investigation of the toxic effects of a Sarcocystis cyst extract following administration via oral intubation, which can mimic the clinical indications associated with feeding as closely as possible. To study the effect of heat treatment on Sarcocystis-infected meat toxicity in liver, we evaluated liver function biomarkers such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) as well, we assessed liver lipid peroxidation and liver histopathology. Brain lipid peroxidation was also assayed to determine the possible presence of brain toxicity. Furthermore, we measured the activities of endogenous antioxidant enzymes such as catalase (CAT) and superoxide dismutase (SOD) to investigate the effects on the antioxidant defense system.
This study was conducted following the ethical protocols for use and care of laboratory animals, and guidelines of the Animal Ethics Committee of the University of Zabol, Zabol, Iran (approval No. 2B-1472).
Macroscopic Sarcocystis cysts in muscle were separated from cattle carcasses in the slaughterhouse. Histopathological examination based on cyst wall diameter confirmed the presence of S. hirsuta. Cysts were detached by using a surgical blade under sterile conditions. Ten percent suspensions of the collected cysts were prepared by homogenizing one gram of the collected cysts in 9 mL of a saline solution. The suspension was centrifuged at 1,500 × g for 15 min at room temperature and then sterilized under UV-light for 3 h with rocking. The supernatant was decanted into 10 mL tubes and brought to a boil in a conical flask at 100℃ for 7 h to create the heat-treated cyst extract. Non-infected meat extracts were prepared by using a method described previously [20]. Non infected meat was provided from an industrial Slaughterhouse center. The histopathological examination was done in order to observe any Sarcocystis infection. Final suspensions were stored in a refrigerator at 4℃ until needed. Prior to conducting the experiments, a 1:10 dilution of the suspension in normal saline was prepared.
Adult male mice (25–32 g), Mus musculus, were housed with free access to sterile tap water and a standard pellet diet. Mice were randomly divided into five experimental groups (n = 8): Group 1 (control group) received normal saline via an oral gavage technique. Group 2 received 0.5 mL/day of the non-infected meat extract by gavage. Group 3 received 0.5 mL/day of freshly prepared S. hirsuta cyst extract by gavage. Group 4 received 0.5 mL/day of a previously frozen S. hirsuta cyst extract. Group 5 mice were given the extract of heat-treated meat infected with S. hirsuta cysts via gavage. All groups were gavaged once per day for 28 days. Extracts were administered by using a metal gavage needle (22-gauge, 2.54–3.81 cm) fitted to a 2 mL syringe. At the end of the experiment, serum samples were collected by using a conventional method and were immediately frozen at −80℃ until use. The daily dose levels were based on our pilot experiments and previous studies [1418].
Serum CAT activity was determined by using the Zell-Bio kit (Zell-Bio, Germany) according to manufacturer's protocol and is expressed as U/mL. Serum SOD activity was measured by using a colorimetric commercial kit (Zell-Bio) according to the manufacturer's instructions. Liver and brain lipid peroxidation levels were measured based on the reaction between malondialdehyde (MDA) and 2-thiobarbituric acid (TBA) by using a spectrophotometer (UNICO UV/VIS-2100 Spectrophotometer; United Products and Instruments, USA) [17]. ALT and AST liver enzymes were measured by using the Pars Azmoon reagent kits (Pars Azmoon, Iran), according to the instructions.
Four weeks of oral gavage feeding of freshly prepared S. hirsuta extract significantly reduced serum CAT activity compared to that in the control group (p < 0.001) (panel B in Fig. 1). There was also a decrease in CAT activity in mice treated with the frozen S. hirsuta extract compared to that in the control group (p < 0.001). Furthermore, serum SOD activities in mice receiving fresh or frozen extracts were significantly lower compared to that in the control mice (p < 0.001 for both) (panel A in Fig. 1). Gavage administration of heat-treated S. hirsuta extract also resulted in reduced CAT and SOD activities when compared to those in the control group (p < 0.01 and p < 0.05, respectively). As expected, serum CAT and SOD activities were not influenced by oral administration of non-infected meat extract (Fig. 1). Further analysis showed that CAT activity in mice receiving fresh S. hirsuta extract was lower than that in the group receiving heat-treated S. hirsuta extract (p < 0.01); however, serum SOD activity did not significantly differ between the two groups (p > 0.05). Furthermore, there were no significant differences in serum SOD and CAT activities between the groups receiving fresh or frozen extracts (p > 0.05) (Fig. 1).
Mice receiving fresh, frozen, and heat-treated S. hirsuta extracts had higher liver MDA levels than that in the control group (p < 0.001, p < 0.001, and p < 0.05, respectively) (panel A in Fig. 2). However, brain MDA levels increased after treatment with fresh and frozen S. hirsuta extracts, but not the heated S. hirsuta extract, compared to that in the control group (p < 0.05 and p < 0.01, respectively) (panel B in Fig. 2). Therefore, oral gavage of heat-treated S. hirsuta extract significantly increased the liver (p < 0.05), but not the brain (p > 0.05), MDA level. Oral administration of the non-infected S. hirsuta extract had no significant effects on the liver and brain MDA levels (p > 0.05). There was also no significant difference in MDA levels between the group treated with fresh extract and the group treated with frozen extract (all p > 0.05).
Our results showed that mice treated with fresh S. hirsuta cyst extract had higher levels of serum ALT and AST compared to those in the control group (p < 0.05 and p < 0.01, respectively) (Fig. 3). There were also significantly higher ALT and AST levels in mice receiving frozen extract compared to those in control mice (p < 0.01). However, there was no significant difference in ALT and AST levels between mice receiving heated extracts and those in the control group (p > 0.05) (Fig. 3).
The liver micrographs of the control group and the group receiving the non-infected extract showed normal morphologies with distinct hepatic cells, a central vein, and radiating sinusoids (panels A and B in Fig. 4). Conversely, liver sections of mice treated with fresh or frozen S. hirsuta extracts demonstrated patterns of cell necrosis (panel C in Fig. 4) and disarrangement of hepatic cords (panel D in Fig. 4). In liver micrographs of mice receiving heat-treated extract, histopathological changes were present, but less prominent (panel E in Fig. 4).
Sarcocystis, one of the most prevalent parasites in muscles of livestock, is responsible for economic and public health burdens worldwide [5], and S. hirsuta is a mildly pathogenic coccidium of cattle [12]. Some studies have reported the presence of Sarcocystis spp. in hamburger meat in Iran; however, there have been no reports of food poisoning caused by this parasite [7816]. Sarcocystis protozoa can be inactivated by cooking or freezing meat products [11]. Regardless, Sarcocystis spp. are intracellular parasites with several antigens and toxic components that may remain intact after being exposed to extreme (high, low) temperatures; therefore, the aim of this study was to examine the possible toxic effects of frozen and heated Sarcocystis extracts in mice in order to elucidate the toxicity of S. hirsuta cysts when meat products are frozen or heated.
Lipid peroxidation is an important mechanism involved in cell membrane destruction and MDA is capable of interacting with amino groups of proteins to form inter-molecular cross-links that inactivate membrane-bound enzymes and receptors. The heat-treated S. hirsuta extract group had increased hepatic MDA levels, distortion of liver architecture, and an insignificant elevation in liver enzyme levels. These results suggest the potential of Sarcocystis cysts to induce lipid peroxidation and liver oxidative damage, probably due to heat-resistant constituents of the cysts.
In contrast to the liver lipid peroxidation results, the brain MDA levels were not influenced by administration of the heat-treated extract. A possible explanation for this difference is that the lipid-soluble constituents in the cyst extract, which are capable of penetrating the blood-brain barrier, have been destroyed by heating.
Liver damage elevates cytosolic enzyme levels, and it has been shown that elevation of liver enzymes can be a marker of liver toxicity [3]. Mice treated with frozen S. hirsuta extract had higher liver enzymes activities, suggesting that the freezing of Sarcocystis-infected meat does not necessarily reduce or eliminate the toxicity of the parasite. Our results support the results of a previous case study that showed elevation of serum liver enzyme levels in patients with acute muscular sarcocystosis [9].
Our study showed depression of enzymatic antioxidant status in the groups treated with fresh, frozen, and heat-treated S. hirsuta extracts. Superoxide radical anion, peroxy radicals, and hydrogen peroxide are known to induce liver fibrosis through the stimulation of type I procollagen synthesis and the generation of reactive aldehyde end-products [21]. Eukaryotic cells are endowed with a broad array of antioxidant defense mechanisms, including enzymatic antioxidant molecules (e.g., CAT, SOD), glutathione peroxidase, and low-molecular-weight scavengers such as beta-carotene, reduced glutathione (GSH), ascorbic acid, vitamin E, and melatonin [10]. In this investigation, the decreases in SOD and CAT might be due to excessive generation of reactive oxygen species or the decreased availability of NADPH, which is required to maintain antioxidant defense system. These results suggest that the S. hirsuta extracts contain oxidizing agents that inhibit the activity of these endogenous antioxidant enzymes.
Taken together, our study suggests that long-term consumption of Sarcocystis-infected meat induces oxidative stress and liver damage, regardless of whether the infected meat is raw or has been frozen or heat treated. However, the results do indicate that heating can partly diminish some aspects of the toxicity of S. hirsuta-infected meat.
Figures and Tables
 | Fig. 1Serum superoxide dismutase (SOD; A) and catalase (CAT; B) activity levels in mice treated with Sarcocystis hirsuta extracts (mean ± SD, n = 8). *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control group. |
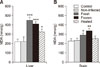 | Fig. 2Malondialdehyde (MDA) levels in liver (A) and brain (B) of mice treated with Sarcocystis hirsuta extracts (mean ± SD, n = 8). *p < 0.05, **p < 0.01, and ***p < 0.001 compared to the control group. |
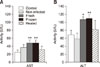 | Fig. 3Serum levels of aspartate aminotransferase (AST; A) and alanine aminotransferase (ALT; B) in mice treated with Sarcocystis hirsuta extracts (mean ± SD, n = 8). *p < 0.05 and **p < 0.01 compared to the control group. |
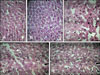 | Fig. 4Photomicrographs of liver sections of the experimental groups. (A) Normal histological appearance of liver tissue of a control mouse. (B) Non-infected extract treated mouse. (C) Fresh extract treated mouse, sinusoidal distention. (D) Liver tissue of a mouse receiving frozen extract, sinusoidal distention. (E) Liver tissue of a mouse received heated extract. H&E stain. 40× (A–E). White arrows, sinusoidal distention; black arrows, the pointer of the microscope. |
Acknowledgments
This study was based on the MS thesis of the first author. We are grateful to the University of Zabol for financial support.
References
1. Al-Hyali NS, Khalil LY, Aljawady MA. Sarcotoxin effect on leukocytic finding and phagocytic activity in mice. J Anim Vet Adv. 2009; 8:2395–2398.
2. Bunyaratvej S, Bunyawongwiroj P, Nitiyanant P. Human intestinal sarcosporidiosis: report of six cases. Am J Trop Med Hyg. 1982; 31:36–41.
3. Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol. 1978; 1:163–171.

4. el-Akkad IN, Mandour AM. On some pharmacological and toxicological effects of a protozoan toxin “sarcocystin”. J Egypt Med Assoc. 1969; 52:942–948.
5. Fayer R, Esposito DH, Dubey JP. Human infections with Sarcocystis species. Clin Microbiol Rev. 2015; 28:295–311.
6. Gjerde B. Molecular characterisation of Sarcocystis bovifelis, Sarcocystis bovini n. sp., Sarcocystis hirsuta and Sarcocystis cruzi from cattle (Bos taurus) and Sarcocystis sinensis from water buffaloes (Bubalus bubalis). Parasitol Res. 2016; 115:1473–1492.

7. Hosseini H, Khaksar R, Shemshadi B. Study on infestation of raw hamburgers to Sarcocystis cyst in Tehran. Iranian Nut Sci. 2007; 4:65–70.
8. Jahed-Khaniki GR, Kia EB. Detection of Sarcocystis cysts from meat supplied for hamburger in Iran by histological method. J Med Sci. 2006; 6:18–21.
9. Lau YL, Chang PY, Tan CT, Fong MY, Mahmud R, Wong KT. Sarcocystis nesbitti infection in human skeletal muscle: possible transmission from snakes. Am J Trop Med Hyg. 2014; 90:361–364.

10. Lei XG, Zhu JH, Cheng WH, Bao Y, Ho YS, Reddi AR, Holmgren A, Arnér ES. Paradoxical roles of antioxidant enzymes: basic mechanisms and health implications. Physiol Rev. 2016; 96:307–364.

11. Lhafi KS, Mitzscherling TA, Kühne M. [Parasites in meat: a challenge for veterinarians in meat hygiene]. Dtsch Tierarztl Wochenschr. 2004; 111:277–281. German.
12. Lindsay DS, Blagburn BL, Braund KG. Sarcocystis spp. and sarcocystosis. Br Med J. 1995; 5:249–254.
13. Lunde MN, Jacobs L. Properties of toxoplasma lysates toxic to rabbits on intravenous injection. J Parasitol. 1964; 50:49–51.

14. Mandour AM. Studies on the toxicity of Sarcocystis. J Med Microbiol. 1969; 2:361–363.
15. Mirzaei M, Rezaei H. A survey on Sarcocystis spp. infection in cattle of Tabriz city, Iran. J Parasit Dis. 2016; 40:648–651.

16. Najafiyan HR, Mohebali M, Keshavarz H. Study on frequency of Sarcocystis spp. by macroscopic and microscopic methods in slaughtered cattle in Shahriar district and their public health importance. Paj Saz. 2007; 77:15–19.
17. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.

18. Saleque A, Bhatia BB, Juyal PD, Rahman H. Toxicity of cyst extract of Sarcocystis fusiformis from buffalo in rabbits and mice. Vet Parasitol. 1991; 38:61–65.

19. Shekarforoush SS, Razavi SM, Abbasvali M. First detection of Sarcocystis hirsuta from cattle in Iran. Iran J Vet Res. 2013; 14:155–157.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download