Abstract
This paper evaluates patient-specific quality assurance (PSQA) in the treatment of small and multiple tumors by the CyberKnife system with fixed collimators, using an ion chamber and EBT3 films. We selected 49 patients with single or multiple brain tumors, and the treatment plans include one to four targets with total volumes ranging from 0.12 cc to 3.74 cc. All PSQA deliveries were performed with a stereotactic dose verification phantom. The A16 microchamber (Standard Imaging, WI, USA) and Gafchromic EBT3 film (Ashland ISP Advanced Materials, NJ, USA) were inserted into the phantom to measure the point dose of the target and the dose distribution, respectively. The film was scanned 1 hr after irradiation by a film digitizer scanner and analyzed using RIT software (Radiological Imaging Technology, CO, USA). The acceptance criteria was <5% for the point dose measurement and >90% gamma passing rate using 3%/3 mm and relative dose difference, respectively. The point dose errors between the calculated and measured dose by the ion chamber were in the range of −17.5% to 8.03%. The mean point dose differences for 5 mm, 7.5 mm, and 10 mm fixed cone size was −11.1%, −4.1%, and −1.5%, respectively. The mean gamma passing rates for all cases was 96.1%. Although the maximum dose distribution of multiple targets was not shown in the film, gamma distribution showed that dose verification for multiple tumors can be performed. The use of the microchamber and EBT3 film made it possible to verify the dosimetric and mechanical accuracy of small and multiple targets. In particular, the correction factors should be applied to small fixed collimators less than 10 mm.
Go to : 
References
1. Yoon J, Park K, Kim JS, Kim YB, Lee H. Acceptance Testing and Commissioning of Robotic Intensity-Modulated Radiation Therapy M6 System Equipped with InCiseTM 2 Multileaf Collimator. Prog Med Phys. 2018; 29(1):8–15.
2. Jang SY, Lalonde R, Ozhasoglu C, Burton S, Heron D, Huq MS. Dosimetric comparison between cone/Iris‐based and InCise MLC‐based CyberKnife plans for single and multiple brain metastases. J Appl Clin Med Phys. 2016; 17(5):1–16.

3. Schmitt D, El Shafie R, Klüter S, et al. Treatment planning for MLC based robotic radiosurgery for brain metastases: plan comparison with circular fields and suggestions for planning strategies. CDBME. 2017; 3(2):151–54.

4. Blanck O, Masi L, Chan MK, et al. High resolution ion chamber array delivery quality assurance for robotic radiosurgery: commissioning and validation. Phys Med. 2016; 32(6):838–46.

5. Kurosu K, Sumida I, Shiomi H, et al. A robust measurement point for dose verification in delivery quality assurance for a robotic radiosurgery system. J Radiat Res. 2017; 58(3):378–85.

6. Palmans H, Andreo P, Christaki K, Huq M, Seuntjens J. Dosimetry of small static fields used in external beam radiotherapy: an IAEA-AAPM international code of practice for reference and relative dose determination. International Atomic Energy Agency, Vienna. 2017.
7. Kim J, Park K, Yoon J, et al. Feasibility Study of a Custom-made Film for End-to-End Quality Assurance Test of Robotic Intensity Modulated Radiation Therapy System. Prog Med Phys. 2016; 27(4):189–95.

8. Ezzell GA, Burmeister JW, Dogan N, et al. IMRT commissioning: multiple institution planning and dosimetry comparisons, a report from AAPM Task Group 119. Med Phys. 2009; 36(11):5359–73.

9. Francescon P, Kilby W, Noll J, Masi L, Satariano N, Russo S. Monte Carlo simulated corrections for beam commissioning measurements with circular and MLC shaped fields on the CyberKnife M6 System: a study including diode, microchamber, point scintillator, and synthetic microdia-mond detectors. Phys Med Biol. 2017; 62(3):1076.

Go to : 
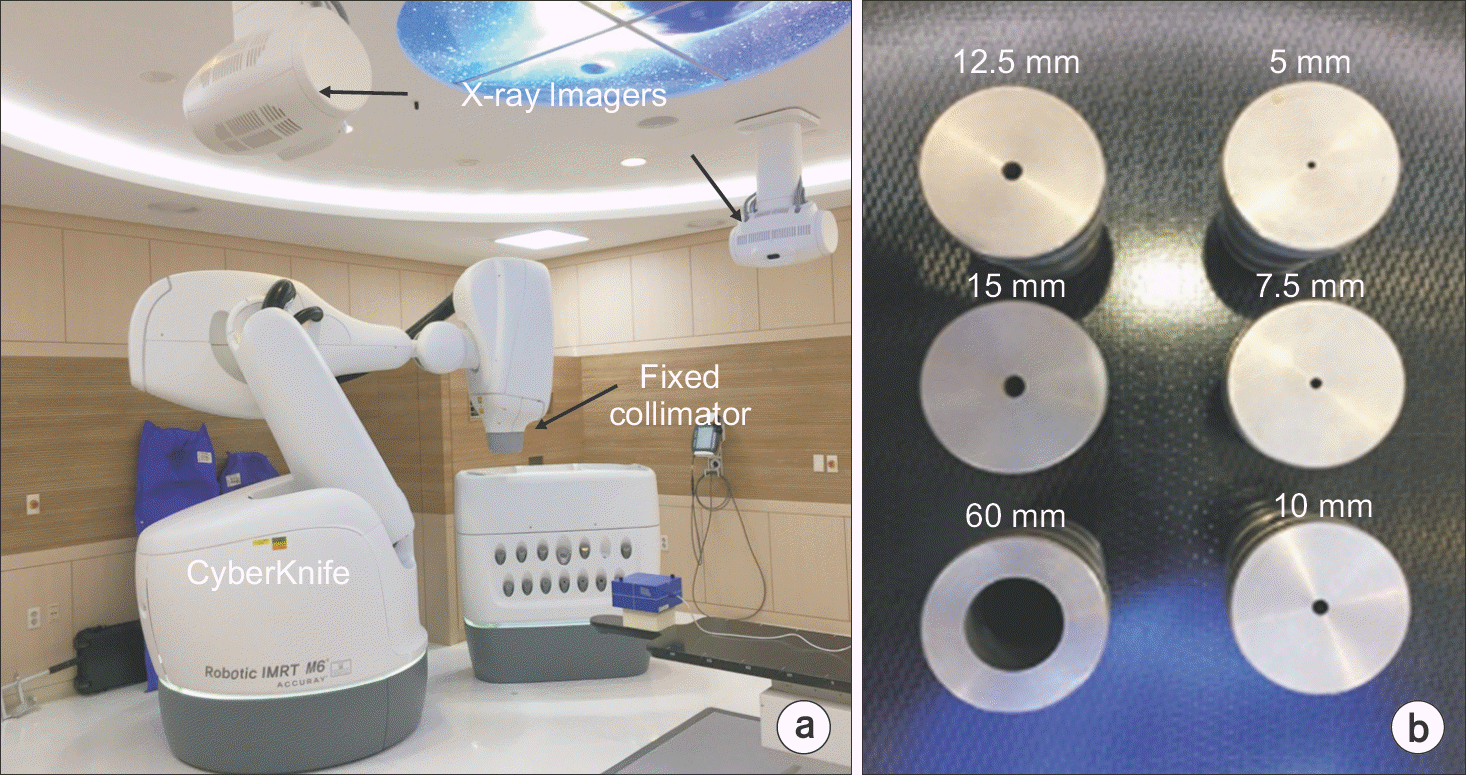 | Fig. 1.Overview of CyberKnife M6 system: (a) Schematics of CyberKnife mounted with the fixed collimator and x-ray live imagers installed on the ceiling and (b) fixed collimators: 5.0, 7.5, 10.0, 12.5 and 15.0 mm for brain tumors. 60 mm for dosimetric quality assurance. |
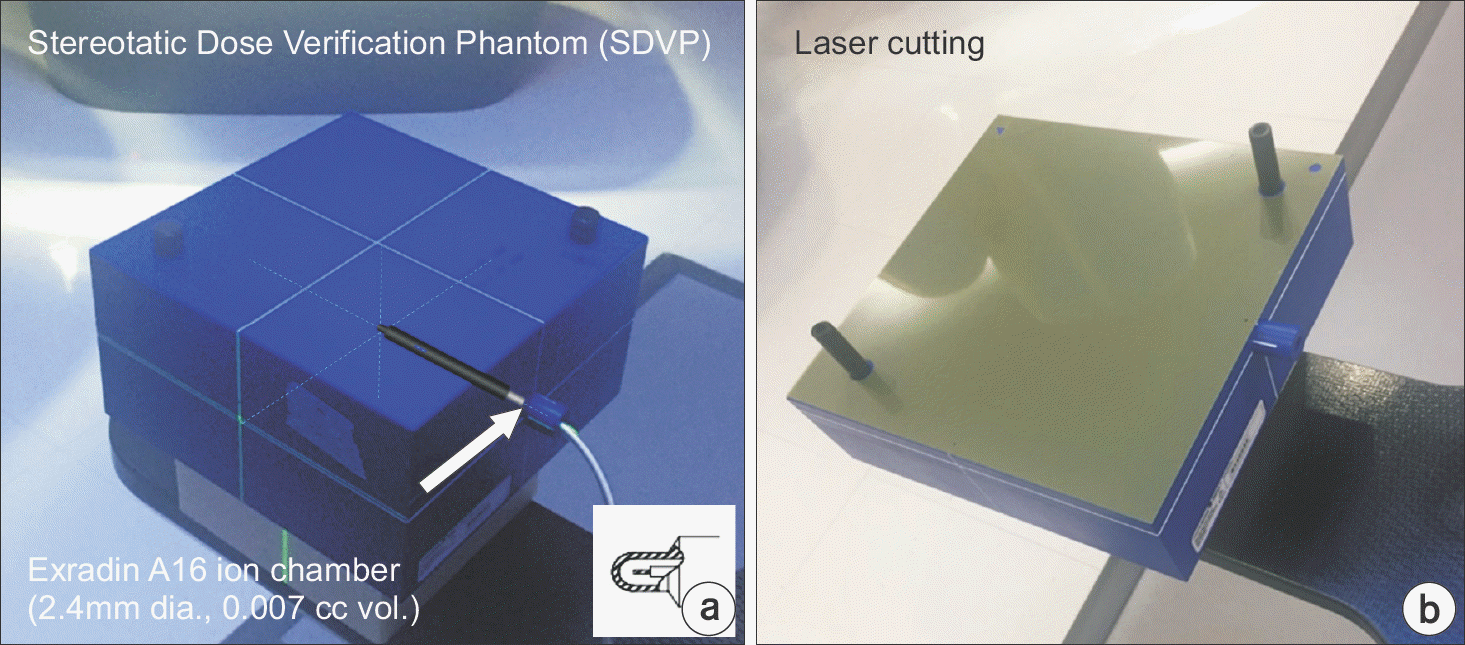 | Fig. 2.PSQA measurement setup: (a) SDVP with Exradin A16 micro-chamber and (b) a customized EBT3 film. |
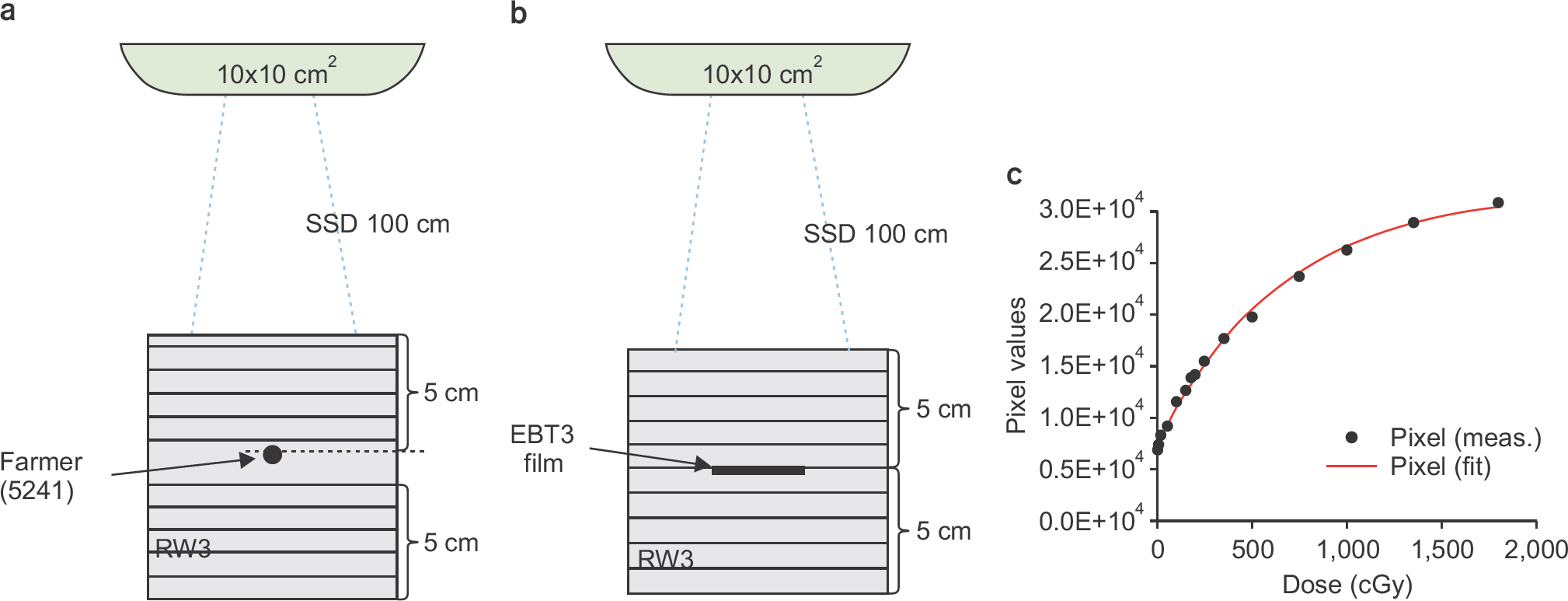 | Fig. 3.Film calibration using RW3 water-equivalent phantom: (a) Absolute dose measurement setup with Farmer chamber, (b) EBT3 film placed at the same position, and (c) the calibration curve for EBT3 film. The measured pixel values were fitted by an exponential function. |
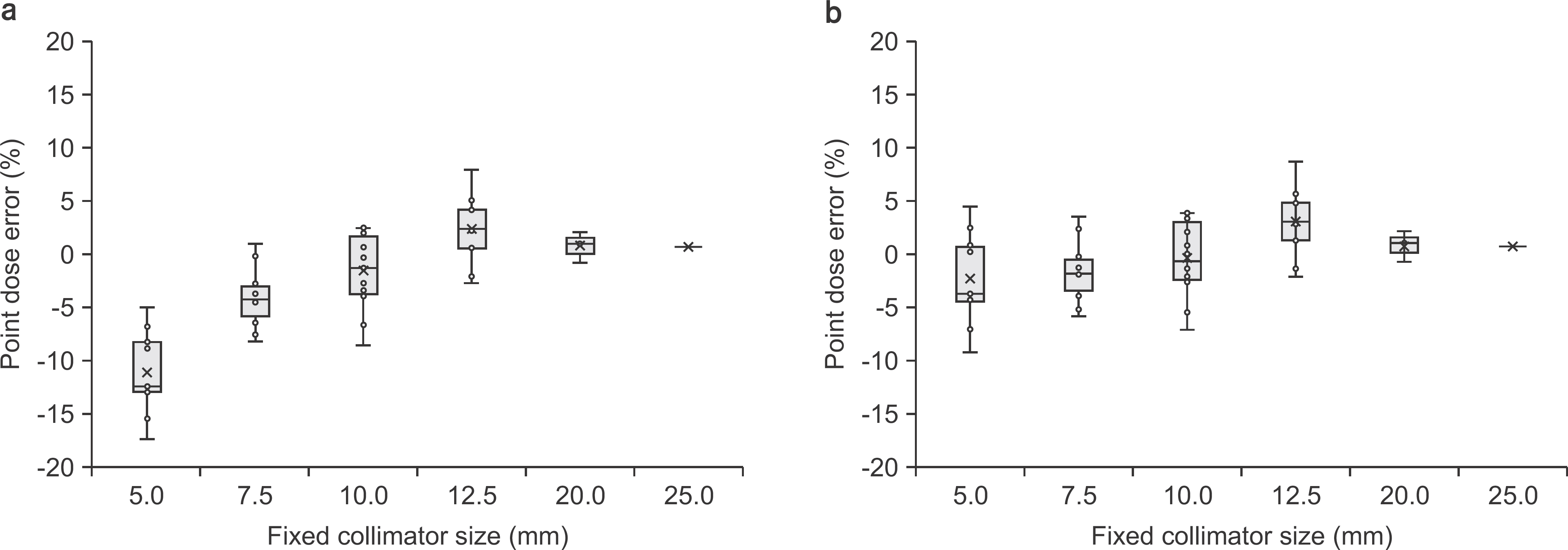 | Fig. 4.Point dose error applied (a) without correction factor and (b) with correction factor by Monte Carlo simulation [10]. Boxes represent the interquartile range and horizontal lines inside each box represent the median. |
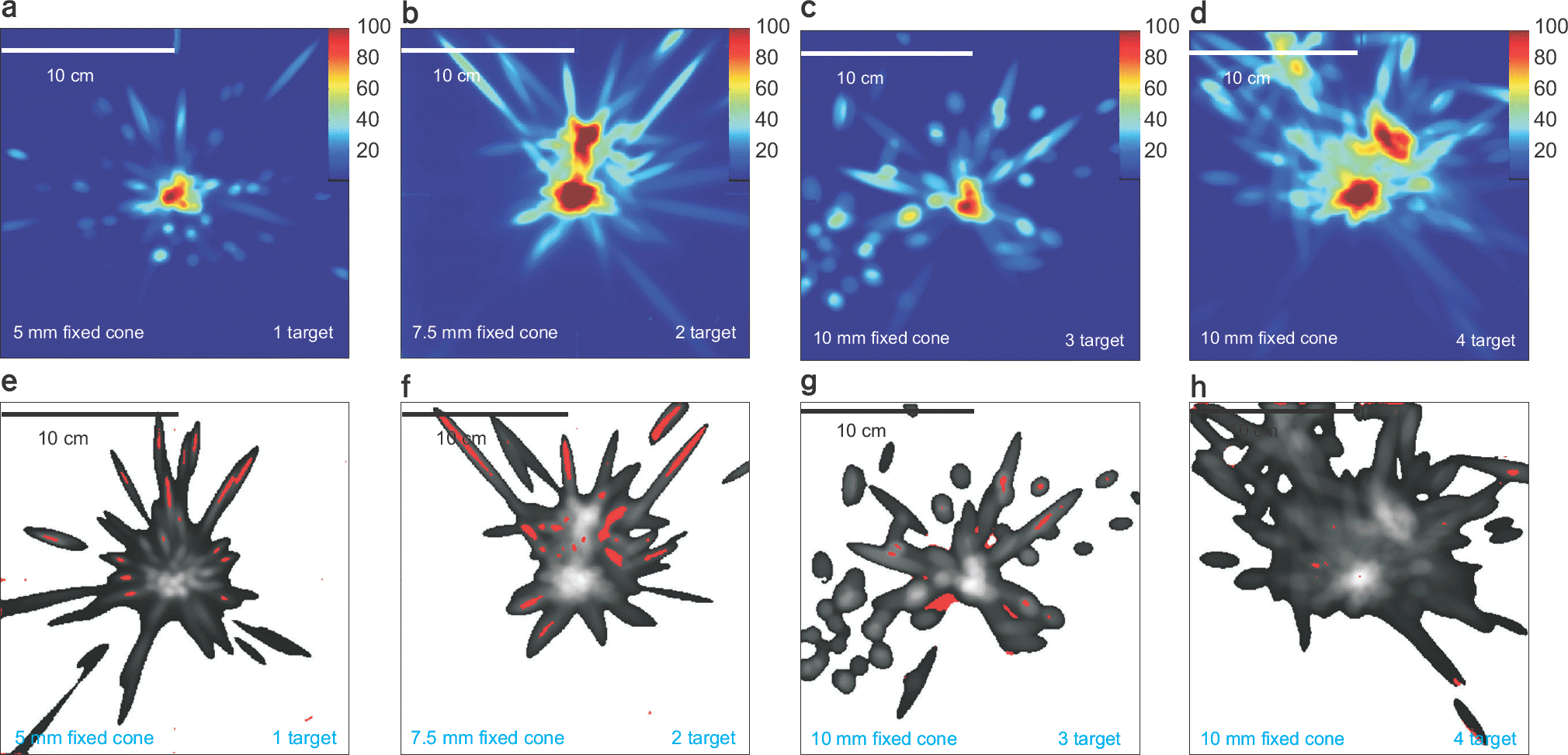 | Fig. 5.Dose (a–d) distributions for CyberKnife plans with various cone sizes and the number of targets and its gamma (e–h) pass/failure (gamma index >1 is red color. (a) and (e) results of 5 mm fixed cone and single target. (b) and (f) 7.0 mm fixed cone and 2 targets. (c) and (g) 10.0 mm and 3 targets, (d) and (h) 10.0 mm fixed cone and 4 targets. |
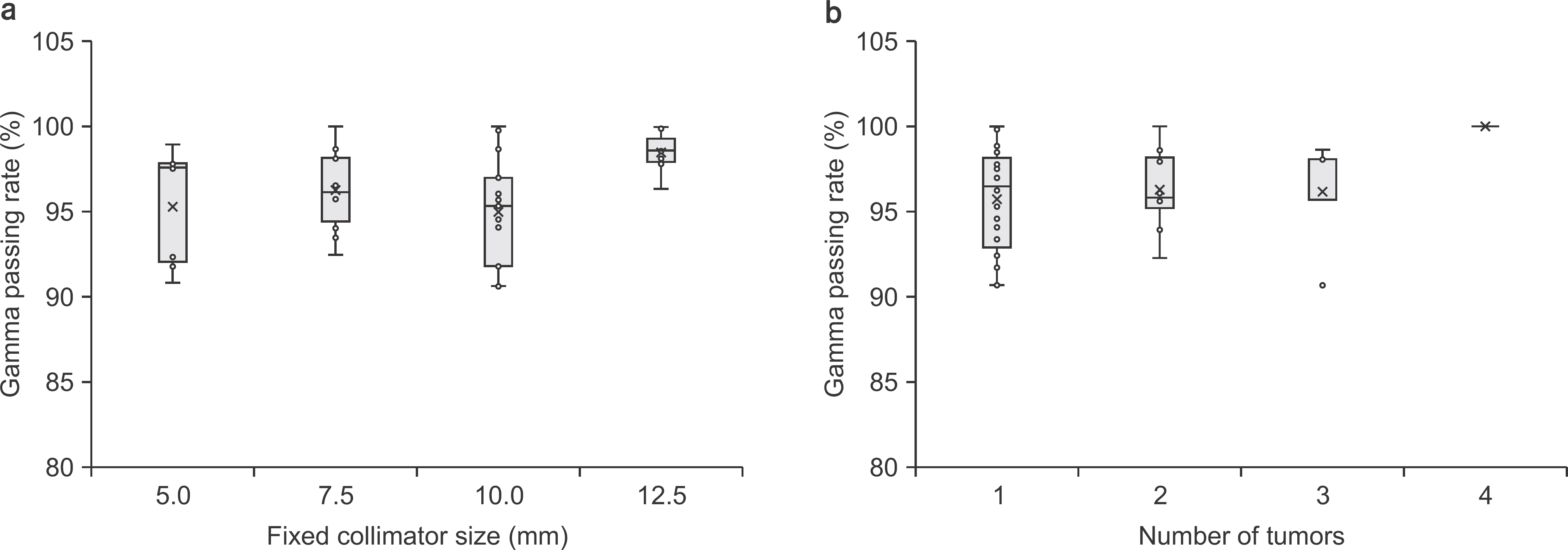 | Fig. 6.Gamma passing rate related to (a) the size of the fixed collimator and (b) the number of tumors. Boxes represent the interquartile range and horizontal lines inside each box represent the median. |
Table 1.
The properties of CyberKnife® plans for brain tumors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download