1. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. 4th ed. Lyon: International Agency for Research on Cancer (IARC);2017.
2. Brathwaite S, Yearsley MM, Bekaii-Saab T, Wei L, Schmidt CR, Dillhoff ME, et al. Appendiceal mixed adeno-neuroendocrine carcinoma: a population-based study of the Surveillance, Epidemiology, and End Results registry. Front Oncol. 2016; 6:148. PMID:
27379210.

3. Šefr R, Němec L, Fabian P, Fiala L. Mixed adenoneuroendocrine carcinoma (MANEC) of the gastrointestinal tract. Rozhl Chir. 2017; 96:41–44. PMID:
28325058.
4. La Rosa S, Marando A, Furlan D, Sahnane N, Capella C. Colorectal poorly differentiated neuroendocrine carcinomas and mixed adenoneuroendocrine carcinomas: insights into the diagnostic immunophenotype, assessment of methylation profile, and search for prognostic markers. Am J Surg Pathol. 2012; 36:601–611. PMID:
22314183.
5. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–321. PMID:
22226517.

6. Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013; 31:1023–1031. PMID:
24142049.

7. Levi Sandri GB, Carboni F, Valle M, Visca P, Garofalo A. Mixed adenoneuroendocrine gastric carcinoma: a case report and review of the literature. J Gastric Cancer. 2014; 14:63–66. PMID:
24765540.

8. Shen C, Chen H, Chen H, Yin Y, Han L, Chen J, et al. Surgical treatment and prognosis of gastric neuroendocrine neoplasms: a single-center experience. BMC Gastroenterol. 2016; 16:111. PMID:
27613657.

9. Shibuya H, Azumi N, Abe F. Gastric small-cell undifferentiated carcinoma with adeno and squamous cell carcinoma components. Acta Pathol Jpn. 1985; 35:473–480. PMID:
2411107.
10. Haratake J, Horie A, Inoshita S. Gastric small cell carcinoma with squamous and neuroendocrine differentiation. Pathology. 1992; 24:116–120. PMID:
1322519.

11. Pericleous M, Toumpanakis C, Lumgair H, Caplin ME, Morgan-Rowe L, Clark I, et al. Gastric mixed adenoneuroendocrine carcinoma with a trilineage cell differentiation: case report and review of the literature. Case Rep Oncol. 2012; 5:313–319. PMID:
22740822.

12. Zhang W, Xiao W, Ma H, Sun M, Chen H, Zheng S. Neuroendocrine liver metastasis in gastric mixed adenoneuroendocrine carcinoma with trilineage cell differentiation: a case report. Int J Clin Exp Pathol. 2014; 7:6333–6338. PMID:
25337287.
13. Bae HI, Lee C, Jo YM, Kwon O, Yu W, Kim MS, et al. Gastric mixed adenoneuroendocrine carcinoma with squamous differentiation: a case report. J Pathol Transl Med. 2016; 50:318–321. PMID:
26755357.

14. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001; 345:725–730. PMID:
11547741.

15. Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol. 2015; 33:3130–3136. PMID:
25559811.

16. Kim KM, Kim MJ, Cho BK, Choi SW, Rhyu MG. Genetic evidence for the multi-step progression of mixed glandular-neuroendocrine gastric carcinomas. Virchows Arch. 2002; 440:85–93. PMID:
11942581.

17. Furlan D, Cerutti R, Genasetti A, Pelosi G, Uccella S, La Rosa S, et al. Microallelotyping defines the monoclonal or the polyclonal origin of mixed and collision endocrine-exocrine tumors of the gut. Lab Invest. 2003; 83:963–971. PMID:
12861036.

18. Scardoni M, Vittoria E, Volante M, Rusev B, Bersani S, Mafficini A, et al. Mixed adenoneuroendocrine carcinomas of the gastrointestinal tract: targeted next-generation sequencing suggests a monoclonal origin of the two components. Neuroendocrinology. 2014; 100:310–316. PMID:
25342539.

19. George J, Walter V, Peifer M, Alexandrov LB, Seidel D, Leenders F, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. 2018; 9:1048. PMID:
29535388.

20. Tang LH, Contractor T, Clausen R, Klimstra DS, Du YC, Allen PJ, et al. Attenuation of the retinoblastoma pathway in pancreatic neuroendocrine tumors due to increased cdk4/cdk6. Clin Cancer Res. 2012; 18:4612–4620. PMID:
22761470.

21. Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016; 18:17. PMID:
26857361.

22. Boscolo-Rizzo P, Da Mosto MC, Rampazzo E, Giunco S, Del Mistro A, Menegaldo A, et al. Telomeres and telomerase in head and neck squamous cell carcinoma: from pathogenesis to clinical implications. Cancer Metastasis Rev. 2016; 35:457–474. PMID:
27501725.

23. Cao Y, Bryan TM, Reddel RR. Increased copy number of the TERT and TERC telomerase subunit genes in cancer cells. Cancer Sci. 2008; 99:1092–1099. PMID:
18482052.

24. Verma R, Sharma PC. Next generation sequencing-based emerging trends in molecular biology of gastric cancer. Am J Cancer Res. 2018; 8:207–225. PMID:
29511593.
25. Janku F, Hong DS, Fu S, Piha-Paul SA, Naing A, Falchook GS, et al. Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors. Cell Reports. 2014; 6:377–387. PMID:
24440717.

26. Blakeley JO, Plotkin SR. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro-oncol. 2016; 18:624–638. PMID:
26851632.

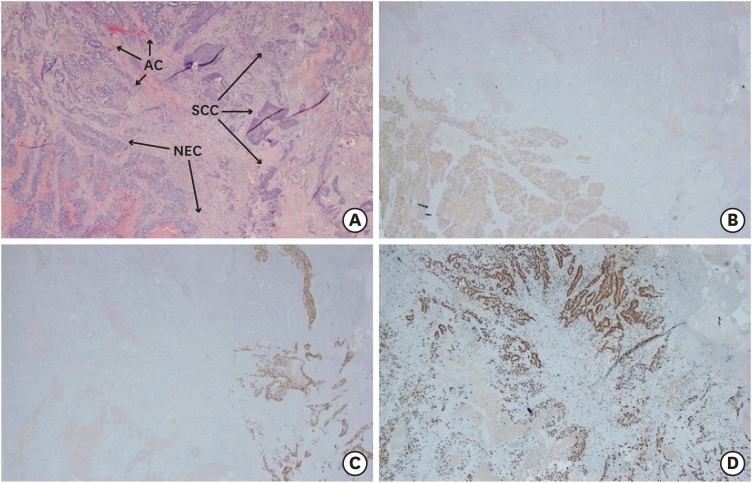
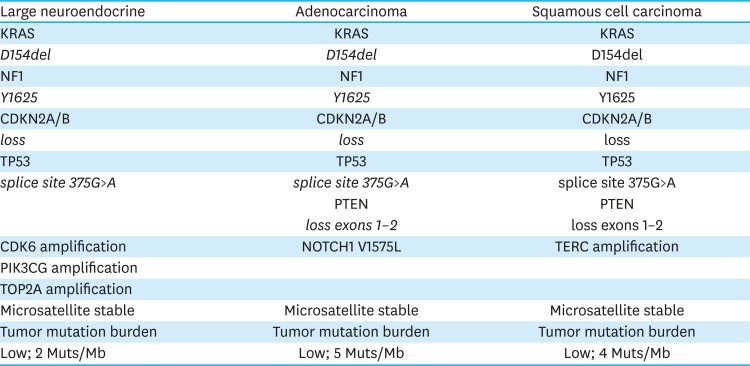
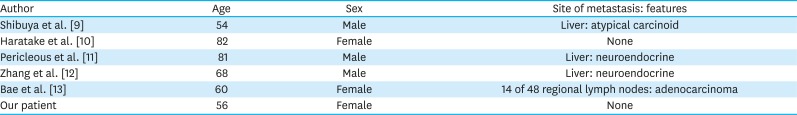
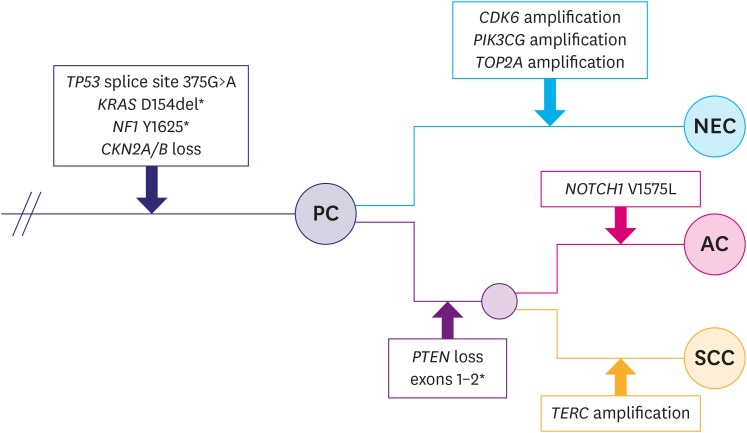




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download