Abstract
Objective
This study examined the all-cause and sex-specific standardized mortality ratios (SMRs) in patients with spondyloarthropathy.
Methods
Studies examining the all-cause and/or cause-specific SMRs in patients with psoriatic arthritis (PsA) and ankylosing spondylitis (AS) compared to the general population were surveyed using MEDLINE, EMBASE, and Cochrane databases and manual searches. A meta-analysis of the all-cause and sex-specific SMRs in patients with rheumatic diseases was then performed.
Results
In total, 7 comparisons (5 PsA and 2 AS) from 6 reports met the inclusion criteria. Disease-specific meta-analysis showed that the pooled SMR was 1.299 (95% confidence interval [CI] 1.092∼1.605, p=0.015) for PsA and 1.784 (95% CI 1.576∼2.020, p<0.001) for AS. Meta-analysis showed that the SMRs of PsA and AS were significantly higher (1.299 to 1.784 times) than those in the general population. The age- and sex-adjusted SMR was highest for AS (1.784), followed by PsA (1.299). Moreover, sex-specific meta-analysis showed that the all-cause SMRs were increased in female and male patients with PsA. On the other hand, mortality increased in male patients with AS (SMR 1.834), whereas there was no significant increase in female patients with AS.
Go to : 
REFERENCES
1. Scarpa R, Mathieu A. Psoriatic arthritis: evolving concepts. Curr Opin Rheumatol. 2000; 12:274–80.

2. Brown MA, Wordsworth BP, Reveille JD. Genetics of ankylosing spondylitis. Clin Exp Rheumatol. 2002; 20(6 Suppl 28):S43–9.
3. Toledano E, Candelas G, Rosales Z, Martínez Prada C, León L, Abásolo L, et al. A meta-analysis of mortality in rheumatic diseases. Reumatol Clin. 2012; 8:334–41.

4. Lee S, Mendelsohn A, Sarnes E. The burden of psoriatic arthritis: a literature review from a global health systems perspective. P T. 2010; 35:680–9. kylosing spondylitis is related to disease activity. Ann Rheum Dis. 2011; 70:1921–5.
6. Yu KH, See LC, Kuo CF, Chou IJ, Chou MJ. Prevalence and incidence in patients with autoimmune rheumatic diseases: a nationwide population-based study in Taiwan. Arthritis Care Res (Hoboken). 2013; 65:244–50.

7. Wong K, Gladman DD, Husted J, Long JA, Farewell VT. Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum. 1997; 40:1868–72.

8. Ali Y, Tom BD, Schentag CT, Farewell VT, Gladman DD. Improved survival in psoriatic arthritis with calendar time. Arthritis Rheum. 2007; 56:2708–14.

9. Buckley C, Cavill C, Taylor G, Kay H, Waldron N, Korendowych E, et al. Mortality in psoriatic arthritis – a single-center study from the UK. J Rheumatol. 2010; 37:2141–4.

10. Mok CC, Kwok CL, Ho LY, Chan PT, Yip SF. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong, China. Arthritis Rheum. 2011; 63:1182–9.

11. Juneblad K, Rantapää-Dahlqvist S, Alenius GM. Disease activity and increased risk of cardiovascular death among patients with psoriatic arthritis. J Rheumatol. 2016; 43:2155–61.

12. Julious SA, Nicholl J, George S. Why do we continue to use standardized mortality ratios for small area comparisons? J Public Health Med. 2001; 23:40–6.

13. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–5.

14. Davey Smith G, Egger M. Meta-analyses of randomised controlled trials. Lancet. 1997; 350:1182.
15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–58.

16. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–34.

17. Nakajima A, Inoue E, Tanaka E, Singh G, Sato E, Hoshi D, et al. Mortality and cause of death in Japanese patients with rheumatoid arthritis based on a large observational cohort, IORRA. Scand J Rheumatol. 2010; 39:360–7.

Go to : 
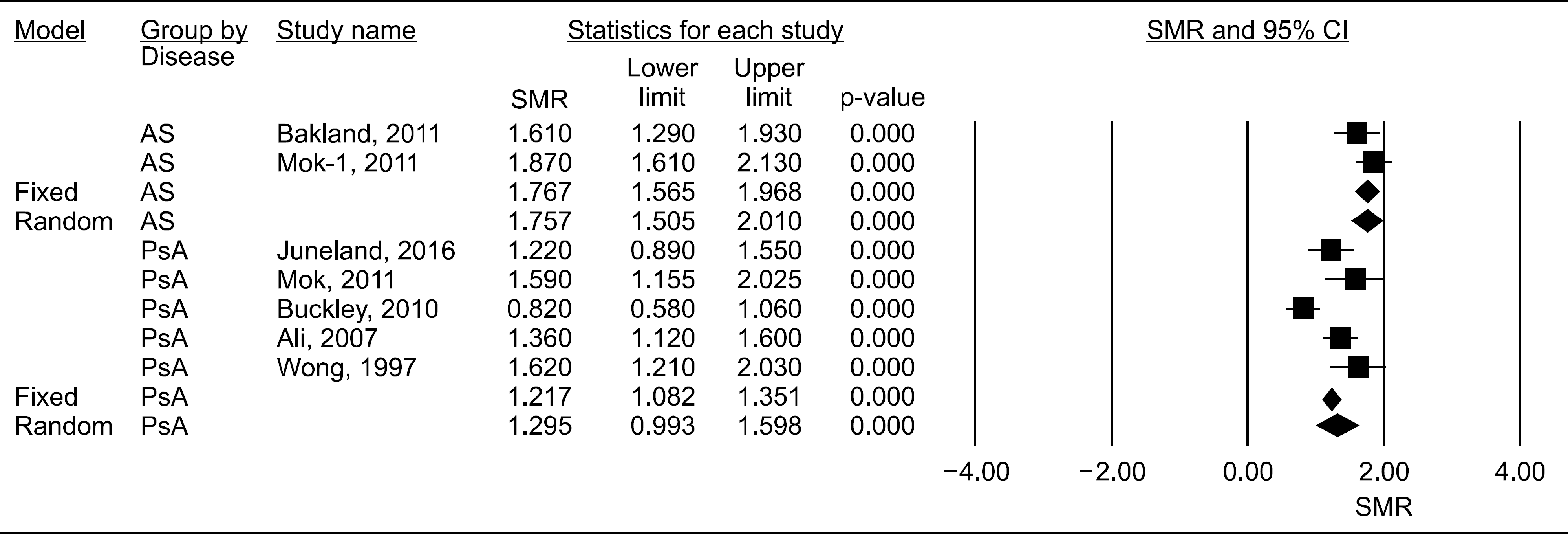 | Figure 2.Meta-analysis of the all-cause standardized mortality ratios in patients with PsA and AS. PsA: psoriatic arthritis, AS: ankylosing spondylitis, SMR: standardized mortality ratio, CI: confidence interval. |
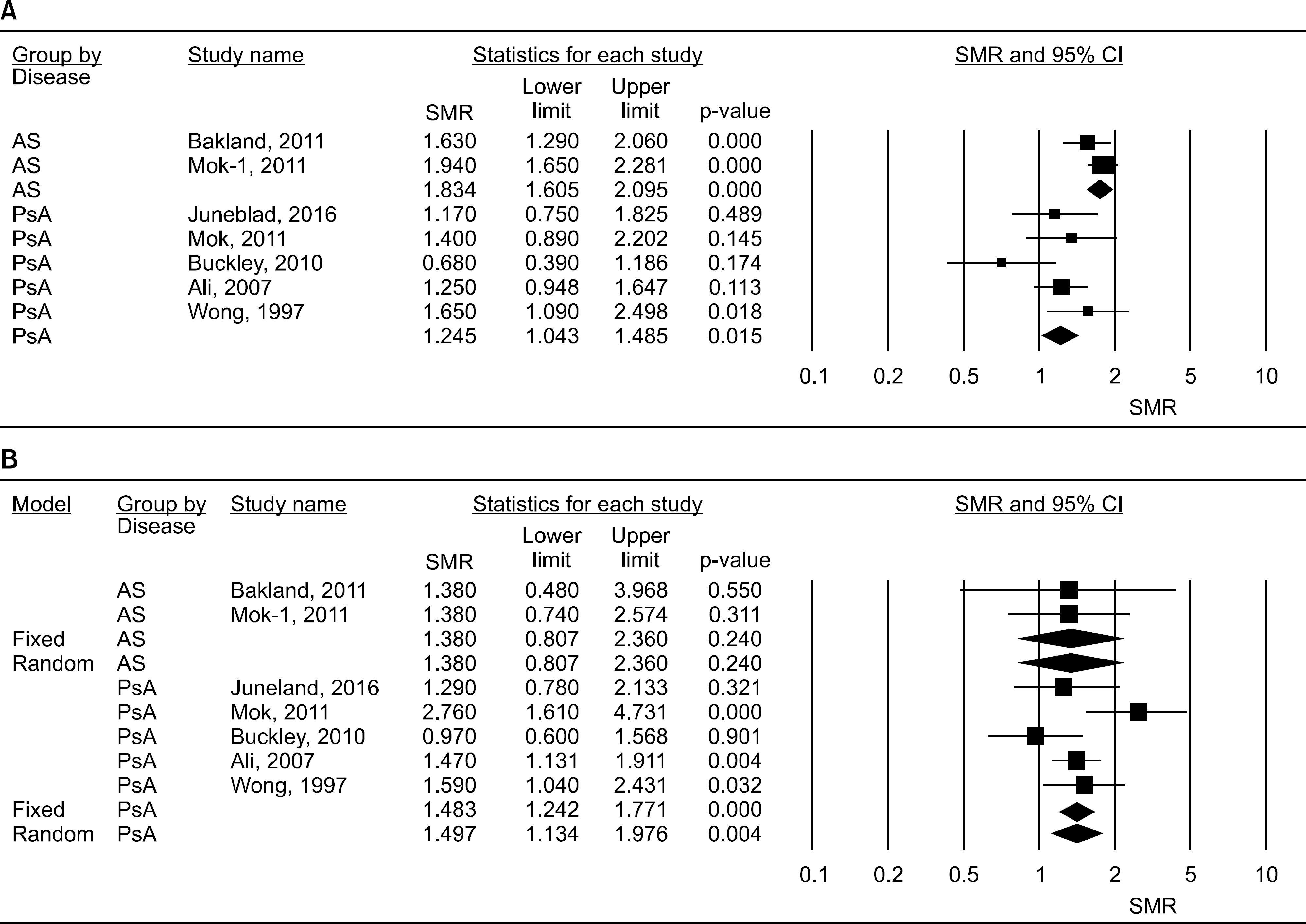 | Figure 3.Meta-analysis of the all-cause standardized mortality ratios in male (A) and female (B) patients with PsA and AS. PsA: psoriatic arthritis, AS: ankylosing spondylitis, SMR: standardized mortality ratio, CI: confidence interval. |
Table 1.
Characteristics of the individual studies included in the meta-analysis
Table 2.
Meta-analysis of the all-cause standardized mortality ratios in patients with PsA and AS




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download