Abstract
Hydroxychloroquine (HCQ) has been used widely for the treatment of several rheumatologic and dermatologic conditions, including systemic lupus erythematosus and rheumatoid arthritis. Its toxic effects on the retina, HCQ retinopathy, is not uncommon among long-term users, and produces characteristic irreversible and progressive outer retinal damage. Recent studies of Asian populations showed different patterns of retinopathy according to ethnicity; for example, a pericentral pattern is more common in Asian populations, whereas the parafoveal type is more prevalent in Caucasian patients. The pericentral pattern, which is common in Asian patients, is likely to lead to a late diagnosis with conventional imaging modalities, thereby necessitating increased attention to the screening of Asian patients. The most recent American Academy of Ophthalmology guidelines suggest optical coherence tomography and a visual field examination as the primary screening tests, and multifocal electroretinogram and fundus autofluorescence as other recommended objective screening tests. The optimal timing and frequency of annual screening depend on the systemic and ocular risk factors. Annual screening should begin from 5 years of drug use in cases without any known risk factors, but patients with major risk factors require earlier regular screening. After a diagnosis of HCQ retinopathy, a decision regarding whether to stop the drug should be made in consultation with the prescribing physician, and the progression of retinopathy should be monitored carefully because the retinopathy can progress even after drug cessation.
Go to : 
REFERENCES
1. Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF. American Academy of Ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016; 123:1386–94.

2. Yusuf IH, Sharma S, Luqmani R, Downes SM. Hydroxychloroquine retinopathy. Eye (Lond). 2017; 31:828–45.

3. Lee DH, Melles RB, Joe SG, Lee JY, Kim JG, Lee CK, et al. Pericentral hydroxychloroquine retinopathy in Korean patients. Ophthalmology. 2015; 122:1252–6.

4. Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015; 122:110–6.

5. Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003; 110:1321–6.
6. Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010; 62:775–84.

7. Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014; 132:1453–60.

8. Ahn SJ, Joung J, Lim HW, Lee BR. Optical coherence tomography protocols for screening of hydroxychloroquine retinopathy in Asian patients. Am J Ophthalmol. 2017; 184:11–8.

9. Leung LS, Neal JW, Wakelee HA, Sequist LV, Marmor MF. Rapid onset of retinal toxicity from high-dose hydroxychloroquine given for cancer therapy. Am J Ophthalmol. 2015; 160:799–805.e1.

10. Navajas EV, Krema H, Hammoudi DS, Lipton JH, Simpson ER, Boyd S, et al. Retinal toxicity of high-dose hydroxychloroquine in patients with chronic graft-versus-host disease. Can J Ophthalmol. 2015; 50:442–50.

11. Browning DJ. The prevalence of hydroxychloroquine retinopathy and toxic dosing, and the role of the ophthalmologist in reducing both. Am J Ophthalmol. 2016; 166:ix–xi.

12. Marmor MF, Hu J. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 2014; 132:1105–12.

13. Mititelu M, Wong BJ, Brenner M, Bryar PJ, Jampol LM, Fawzi AA. Progression of hydroxychloroquine toxic effects after drug therapy cessation: new evidence from multimodal imaging. JAMA Ophthalmol. 2013; 131:1187–97.
14. Ahn SJ, Ryu SJ, Lim HW, Lee BR. Toxic effects of hydroxychloroquine on the choroid: evidence from multimodal imaging. Retina. 2018; DOI: DOI: 10.1097/IAE.000000000-0002047.
15. Kellner S, Weinitz S, Farmand G, Kellner U. Cystoid macular oedema and epiretinal membrane formation during progression of chloroquine retinopathy after drug cessation. Br J Ophthalmol. 2014; 98:200–6.

16. Hong EH, Ahn SJ, Lim HW, Lee BR. The effect of oral aceta-zolamide on cystoid macular edema in hydroxychloroquine retinopathy: a case report. BMC Ophthalmol. 2017; 17:124.

17. Ahn SJ, Ryu SJ, Joung JY, Lee BR. Choroidal thinning associated with hydroxychloroquine retinopathy. Am J Ophthalmol. 2017; 183:56–64.

18. Browning DJ. Hydroxychloroquine and chloroquine retinopathy. New York: Springer;2014. p. 219.
Go to : 
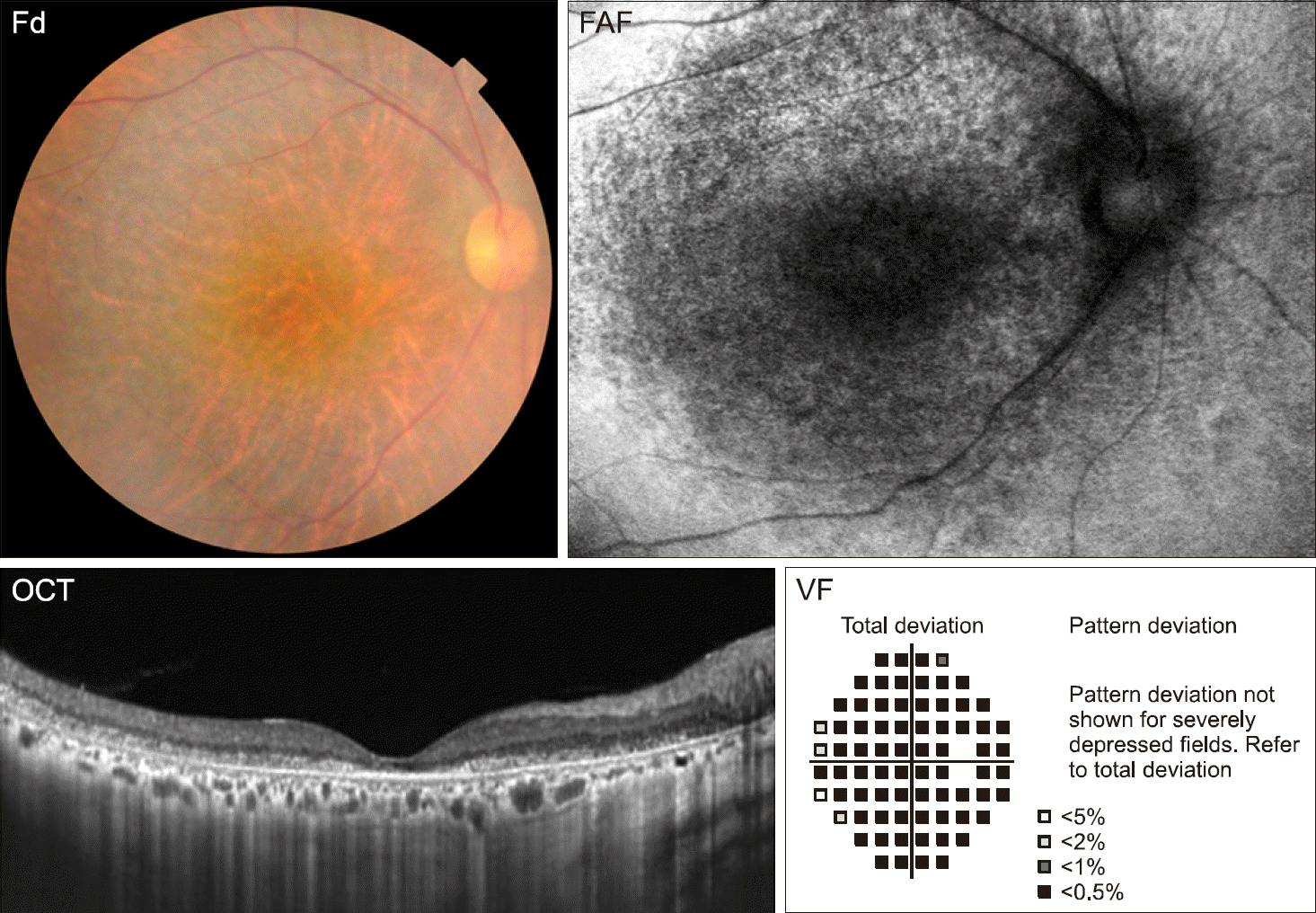 | Figure 1.Fundus photograph (Fd), optical coherence tomography (OCT), fundus autofluorescence (FAF), and visual field (VF) examination of the right eye in a 54-year-old female systemic lupus erythematosus patient with hydroxychloroquine retinopathy. Retinal pig-mentary changes observable in the fundus photograph corre-spond to outer retinal damage visible on the OCT image, hy-po-autofluorescence on FAF, and complete visual field loss in visual field examination. The visual acuities in both eyes were “hand motion.” |
Table 1.
Screening for hydroxychloroquine retinopathy recommended for Asian patients with hydroxychloroquine use
Table 2.
Diagnostic ability, sensitivity, and specificity of the recommended screening tests for hydroxychloroquine retinopathy [18]
| Examinations | Sensitivity | Specificity |
|---|---|---|
| 10-2 VF examination | 85.7 | 92.5 |
| Spectral-domain OCT | 78.6 | 98.1 |
| Multifocal electroretinogram | 92.9 | 86.9 |
| Spectral-domain OCT and 10-2 VF examination | 85.7 | 92.5 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download