This article has been
cited by other articles in ScienceCentral.
Dear Editor,
The genus
Skermanella, comprising gram-negative, motile, and pigmented bacteria, was first described in 1999 [
1]. However, human infections caused by
Skermanella have not been reported to date [
23]. Here, we present a case of necrotizing fasciitis caused by
Skermanella sp. This study was exempted by the Institutional Review Board of Jeju National University Hospital (IRB No: JNUH 2018-04-005).
A 76-year-old man with a 13-year history of hypertension and a 6-year history of diabetes mellitus was transferred to a tertiary-care hospital (Jeju National University Hospital, Jeju, Korea) from a local clinic after experiencing pain in the left lower limb and hypotension for one day. The patient resided in a rural area and had consumed fresh fish at a Japanese restaurant, five days prior to the appearance of his presenting symptoms. On admission, his vital signs were as follows: blood pressure, 66/50 mmHg; pulse rate, 105/minutes; respiratory rate, 31/minutes; and body temperature, 38.7℃. On physical examination, his mental status was alert, and no specific findings were observed except for swelling and tenderness of the left lower extremity, and small vesicles and redness on the left ankle (
Fig. 1A). Gram staining of aspiration fluid from the vesicle on the skin was negative.
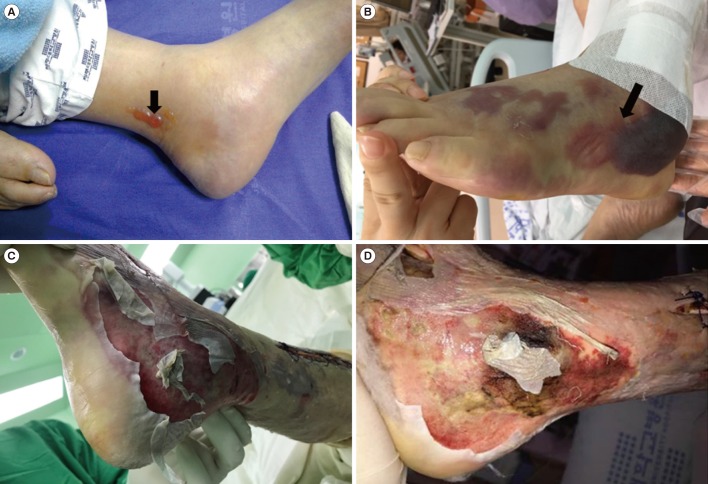 | Fig. 1Development of infected area. Swelling and small vesicles (A, black arrow) detected on the left ankle area of the patient on presentation to the emergency department. The lesions changed to hemorrhagic bullae (B, black arrow) extending from the foot to shin area within about 12 hours after admission. In the operating room, the skin of the left foot detached easily along the lateral aspect (C) with progression to an ischemic color change (D) along the medial aspect.
|
The patient was admitted to the intensive care unit because vasopressor administration was required to maintain blood pressure. The following morning, about 12 hours after admission, the patient reported more severe pain in his left leg. Upon examination, a more aggravated color change and extended hemorrhagic bullae were observed (
Fig. 1B). Lower extremity computed tomography showed subcutaneous swelling without an abscess. Based on suspicion of
Vibrio sepsis, intravenous ceftriaxone (2 g/day) and ciprofloxacin (800 mg/day) were initiated, and fasciotomy was immediately performed on the lower leg (
Fig. 1C and 1D). The patient was further administered various antibiotics such as ceftazidime, amikacin, cefepime, doxycycline, and tigecycline. He was discharged on the 86th day after admission, when complete blood count and biochemistry results had returned to the normal range, and the patient had recovered completely.
Cultures of two sets of blood samples, bullae aspiration fluid, and debrided tissue were performed using thiosulfate citrate bile salts sucrose medium for three weeks. The samples were stored at −20℃ till subsequent use. Two peripheral blood samples (about 5 mL), one in an EDTA tube and another in a polyethylene bottle, as well as vesicle and tissue samples (about 5 mg each) were also tested using reverse transcription- PCR targeting the
hly,
tlh, and
vvhA genes [
45]; PCR results were the same as those for
Vibrio vulnificus (not shown). However, conventional biochemical tests, performed three times, failed to identify this isolate to the species level. Repeated culture tests also showed no growth.
We next attempted to amplify the 16S rRNA gene from the blood, vesicles, and tissue samples [
5]. The determined 16S rRNA gene sequences, both 1,283 bp, were identical, and showed the highest similarity (99.84%) with
Skermanella aerolata KACC 11604
T, followed by
Skermanella stibiiresistens SB22
T (97.58%) and
Skermanella parooensis ACM 2042
T (97.48%), based on analysis using the EzBioCloud database (
www.ezbiocloud.net) [
6]. A phylogenetic tree constructed by the neighbor-joining method (MEGA 5.10) confirmed that the causative pathological bacterium in the tissue and serum was the closest to
S. aerolata (
Fig. 2). The 16S rRNA gene sequences were deposited in the GenBank nucleotide sequence database under accession numbers MH398562 and MH410495.
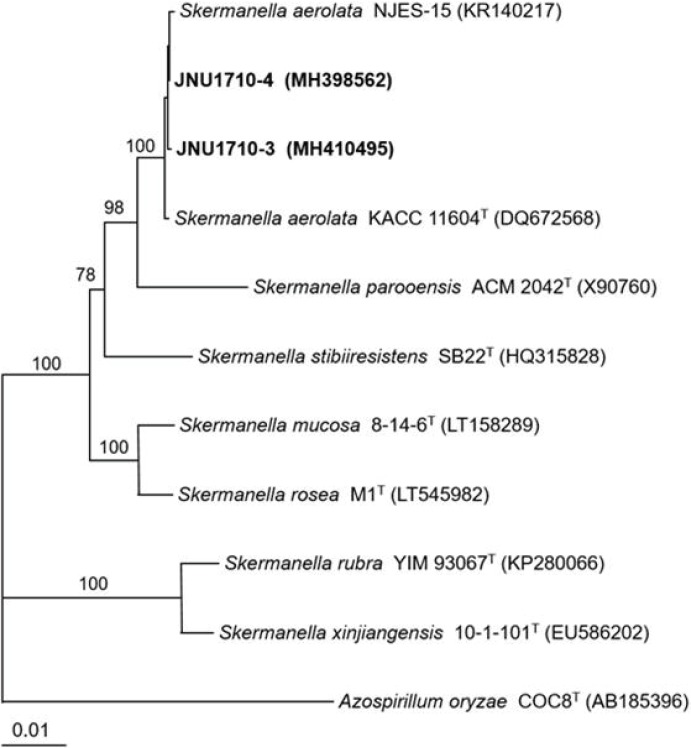 | Fig. 2Phylogenetic tree of isolates JNU1710-3 (from tissue) and JNU1710-4 (from serum) and species of Skermanella based on 16S rRNA gene sequences. The tree was reconstructed by the neighbor-joining method (MEGA 5.10), and Azospirillium oryzae COC8T was used as an outgroup. Numbers on branching nodes are percentages of 1,000 bootstrap replications; only values ≥ 50% are shown. The scale bar represents one substitution per 100 nucleotides.
|
To our knowledge, this represents the first case of human infection caused by a
Skermanella species.
S. aerolata was first isolated from the air of Asian Dust in 2007 [
7], and is known to shape the bacterial community in soil or soda lime [
38]. Thus, the patient might have become infected with
S. aerolata by contact with soil. However, we cannot exclude the possibility that the fish he consumed was contaminated with the bacterium.
In this case,
S. aerolata caused necrotizing fasciitis, a very dangerous infectious disease. Necrotizing soft-tissue infections and sepsis may be caused by any bacterium, but
V. vulnificus and
Aeromonas species are associated with particularly severe cases (we did not test for
Aeromonas sp.) [
9]. The patient showed rapidly progressing hemorrhagic bullae and shock. Moreover, because he had recently consumed seafood,
Vibrio infection was suspected, which has been reported frequently on Jeju Island, Korea [
10]. Thus, he was treated with antibiotics and surgery as rapidly as possible.
Since S. aerolata was identified only by 16S rRNA gene sequencing, we could not obtain data regarding its antibiotic susceptibility. However, S. aerolata infection is assumed to require treatment with broad-spectrum antibiotics, and early surgery was likely crucial to the patient's treatment in this case.
In summary, we reported that a bacterium present in the soil or air of Asian Dust may cause potentially fatal infectious disease. Skermanella species such as S. aerolata may be a cause of human infections that can mimic the signs of Vibrio infections.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download