Abstract
Frequencies of red blood cell (RBC) blood group antigens differ by ethnicity. Since the number of immigrants is increasing in Korea, RBC antigens should be assessed in children/youths with parents of different ethnicities to ensure safe transfusions. We investigated the frequency of RBC antigens, except for ABO and RhD, in 382 children and youths with parents having Korean and non-Korean ethnicities. Subjects were divided into those with ethnically Korean parents (Korean group; N=252) and those with at least one parent of non-Korean ethnicity (non-Korean group; N=130). The 37 RBC antigens were genotyped using the ID CORE XT system (Progenika Biopharma-Grifols, Bizkaia, Spain). The frequencies of the Rh (E, C, e, hrS, and hrB), Duffy (Fya), MNS (Mia), and Cartwright (Ytb) antigens differed significantly between the two groups. Eight and 11 subjects in the Korean and non-Korean groups, respectively, exhibited negative expression of high-frequency antigens, whereas 14 subjects in the non-Korean group showed positive expression of low-frequency antigens. The frequency of RBC antigens has altered alongside demographic changes in Korea and might lead to changes in distribution of RBC antibodies that cause acute or delayed hemolytic transfusion reaction.
Red blood cell (RBC) alloimmunization is acquired when a person is exposed to an RBC antigen (except for the ABO blood group) through blood transfusion, transplantation, or during pregnancy. Alloimmunization potency differs by blood type and exposure source [1]. Of the >300 RBC antigens [2], clinically significant alloantibodies (those against the Rh, MNS, Kell, Duffy, and Kidd blood group antigens) can cause hemolytic transfusion reaction and hemolytic disease in the fetus and newborn [3].
The frequencies of RBC blood group antigens differ by ethnicity. Accordingly, a change in the ethnicity distribution of a population will lead to altered RBC antigen expression [1]. Transfusion laboratories utilize molecular testing of C/c, E/e, K/k, Kpa/Kpb, Jsa/Jsb, Jka/Jkb, Fya/Fyb, MN, S/s, Lua/Lub, Dia/Dib, Coa/Cob, Doa/Dob, Joa, Hy, LWa/LWb, Sc1/Sc2, and other antigens for targeted blood donor recruitment to provide transfusion support for ethnically diverse patient populations [2]. In recent years, multiple molecular testing platforms have been used to predict phenotypes based on blood group genetics [456]. The ID CORE XT system (Progenika Biopharma-Grifols, Bizkaia, Spain) can be used to simultaneously identify multiple allelic variants encoding the most important RBC antigens. This system has received the Conformité Européenne label, and its ability to accurately identify RBC antigens in various ethnic groups has been well established [789].
Several studies have reported the phenotyping and genotyping of blood groups in Korean adults [101112]. However, the number of immigrants to Korea has been increasing; the proportion of foreign residents among the total Korean population has risen from 1.9% in 2004 to 4.0% in 2016 [13]. The cumulative number of interethnic marriages has also been growing; 93,786 and 152,374 marriages involving immigrants occurred in Korea in 2006 and 2016, respectively [13]. Therefore, up-to-date information on RBC antigens in non-Korean adults, children, and youths is needed to reduce the risk of RBC alloimmunization.
This prospective and observational multi-center study investigated the frequency of RBC antigens, except for ABO and RhD, in children and youths with ethnically Korean and non-Korean parents, using molecular typing, and assessed the characteristics of the RBC antigens.
We recruited a total of 382 healthy volunteers and patients aged <30 years from September 2015 to August 2017 at seven training hospitals in Korea. The subjects or their parents identified parental ethnicities and were divided into the Korean group (both parents born in Korea and of Korean ethnicity; N=252) and non-Korean group (at least one parent born outside Korea and of non-Korean ethnicity; N=130). Of the latter group, 85.3% had one or both parents of Southeast or Chinese, Japanese, and Mongolian ethnicity. The subjects' general characteristics are described in Table 1. No differences were observed between the two groups, except for age.
The study protocol was approved by the institutional review board of the Pusan National University Hospital (H-1509-001-033), and written informed consent was obtained from all subjects or their guardians.
Genomic DNA was extracted from whole blood and supplemented with EDTA, using the QuickGene DNA whole blood kit S (Kurabo Industries Ltd., Osaka, Japan), according to the manufacturer's protocol. The DNA samples were frozen at −80℃ until analyzed using the ID CORE XT assay. The ID CORE XT system, by Luminex 100 Instrument (Luminex, Austin, TX, USA) based on the Luminex xMAP technology, was used to genotype 37 RBC antigens. The raw data were processed with the ID CORE XT Analysis Software to obtain the genotypes as well as the predicted blood group phenotypes based on the published literature. Antigens with a negative expression frequency <1.0% and >99.0% in the Korean group were defined as high- and low-frequency antigens, respectively [1].
Continuous variables were expressed as medians and ranges. Comparisons were performed using Pearson's chi-squared test and Fisher's exact test. Data were analyzed using IBM SPSS Statistics 22 (IBM Corp., Armonk, NY, USA). P<0.05 was considered statistically significant.
RBC antigen genotype frequencies in Korean and non-Korean subjects are shown in Table 2. Nine “no calls” were observed in the Lutheran blood group (low signal or indeterminate genotype in LU:c.230A>G); these did not resolve following retesting.
The frequencies of the predicted phenotypes of the RBC antigens in Korean and non-Korean subjects are shown in Table 3. The frequency of most phenotypes was similar in Korean and non-Korean subjects; however, the positive predicted phenotype frequencies of the Rh (C, E, e, hrS, and hrB), Duffy (Fya), MNS (Mia), and Cartwright (Ytb) antigens differed (P<0.05). We observed a higher frequency of C, e, hrS, hrB, Mia, and Ytb, as well as a lower frequency of E and Fya in non-Korean subjects (Table 3).
Regarding high-frequency antigens, the expression of Duffy (Fya), Diego (Dib), and Dombrock (Dob) was not detected in three (1.2%), one (0.4%), and four (1.6%) Korean subjects, respectively, and in seven (5.4%), one (0.8%), and one (0.8%) non-Korean subjects, respectively. Expression of MNS(s) was not observed in two (1.5%) non-Korean subjects but was observed in all Korean subjects (Table 3).
Regarding low-frequency antigens, Kell (K, Jsa), MNS (Mia), Cartwright (Ytb), and Lutheran (Lub) were not expressed in Korean subjects, but were expressed in two (1.5%), one (0.8%), seven (5.3%), three (2.3%), and one (0.8%) non-Korean subjects, respectively (Table 3).
To the best of our knowledge, this study is the first to report the frequency of RBC antigens in children and youths with interethnic parents in Korea, using molecular typing. While the results for Korean subjects were similar to those reported in other studies [101112], we identified significant differences in the frequency rates of Rh, Duffy, MNS, and Cartwright antigens between Korean and non-Korean subjects.
It is likely that some transfusion recipients produce antibodies thus far rarely identified in Korea. When children/youths with a non-Korean parent donate blood at an appropriate age, Korean recipients are at risk of producing anti-K, anti-Jsa, anti-Mia, anti-Ytb, or anti-Lub antibodies. When children/youths with a non-Korean parent receive blood products in Korea, they are at risk of producing anti-Fya, anti-s, anti-Dib, or anti-Dob antibodies. There might also be changes in the frequencies of Rh antibodies, which are associated with changes in the expression frequencies of the E, C, and e antigens. Changes in the frequencies of antibodies against antigens of the Rh, Kell, and Duffy blood group systems are particularly important because they have high immunogenicity [14].
The number of transfusion recipients with negative expression of high-frequency antigens and of blood donors with positive expression of low-frequency antigens will likely increase in Korea because of demographic changes in the population. Our findings show that the frequency of the clinically significant alloantibodies anti-K, anti-E, anti-C, anti-e, and anti-Fya will alter in cases of transfusions and transplantations and in pregnant women in Korea. As demographic changes will likely continue in Korea, the frequency of the major RBC antigens should be assessed continuously.
The limitation of this study is that the ethnic distribution of Korean nationals and foreign residents in Korea was not consistent. Because the effect on RBC antigens of each ethnic group may differ, it is necessary to conduct a large-scale study, in which the number of foreign residents from each ethnic group is consistent with the number of Korean nationals.
Acknowledgment
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (funding code 2016-E830003-00).
References
1. Han KS, Park KU, et al. Transfusion medicine. 4th ed. Seoul: Korean Medical Book Publisher;2014. p. 184–191.
2. Shafi H, Abumuhor I, Klapper E. How we incorporate molecular typing of donors and patients into our hospital transfusion service. Transfusion. 2014; 54:1212–1219. PMID: 24628032.
3. Surendran SK, Allard S, Regan F. The management of third and fourth degree perineal tears. Royal College of Obstetricians and Gynaecologists Green Top Guidelines No 65. 2014. Updated on Jun 2018. https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-65.pdf.
4. Boccoz SA, Blum L, Marquette C. DNA biosensor/biochip for multiplex blood group genotyping. Methods. 2013; 64:241–249. PMID: 24080420.
5. Liu Z, Liu M, Mercado T, Illoh O, Davey R. Extended blood group molecular typing and next-generation sequencing. Transfus Med Rev. 2014; 28:177–186. PMID: 25280589.
6. Goldman M, Núria N, Castilho L. An overview of the Progenika ID CORE XT: an automated genotyping platform based on a fluidic microarray system. Immunohematology. 2015; 31:62–68. PMID: 26495891.
7. Goldman M, Cemborain A, Cote J, El Hamss R, Flower RL, Garaizar A, et al. Identification of six new RHCE variant alleles in individuals of diverse racial origin. Transfusion. 2016; 56:244–248. PMID: 26435076.
8. Finning K, Bhandari R, Sellers F, Revelli N, Villa MA, Muñiz-Díaz E, et al. Evaluation of red blood cell and platelet antigen genotyping platforms (ID CORE XT/ID HPA XT) in routine clinical practice. Blood Transfus. 2016; 14:160–167. PMID: 26674823.
9. López M, Apraiz I, Rubia M, Piedrabuena M, Azkarate M, Veldhuisen B, et al. Performance evaluation study of ID CORE XT, a high throughput blood group genotyping platform. Blood Transfus. 2018; 16:193–199. PMID: 27893355.
10. Kim KH, Kim BR, Choi JL, Woo KS, Kim JM, Han JY. Difference of Rh phenotype between irregular antibody positive patients and rhd positive population in Korea. Korean J Blood Transfus. 2014; 25:60–68.
11. Lee NY, Suh JS, Ryang DW, Son HC, Kwon KC, Yoo BJ. Discrepant frequency of Rh subtype and Kell blood group antigens between Korean pregnant women and their neonates. Korean J Blood Transfus. 1998; 9:37–43.
12. Hong YJ, Chung Y, Hwang SM, Park JS, Kwon JR, Choi YS, et al. Genotyping of 22 blood group antigen polymorphisms and establishing a national recipient registry in the Korean population. Ann Hematol. 2016; 95:985–991. PMID: 27021300.
13. IT Strategy & Management Division of Ministry of Justice. Foreign residents. Korea Immigration Services Statistics 2016. Statistics Korea;2016. p. 38–39.
14. Laura C, Theresa D. Immunohematology. In : Richard M, Matthew R, editors. Henry's clinical diagnosis and management by laboratory methods. 22nd ed. Philadelphia: Elsevier Saunders;2011. p. 675–710.
Table 1
Subject characteristics
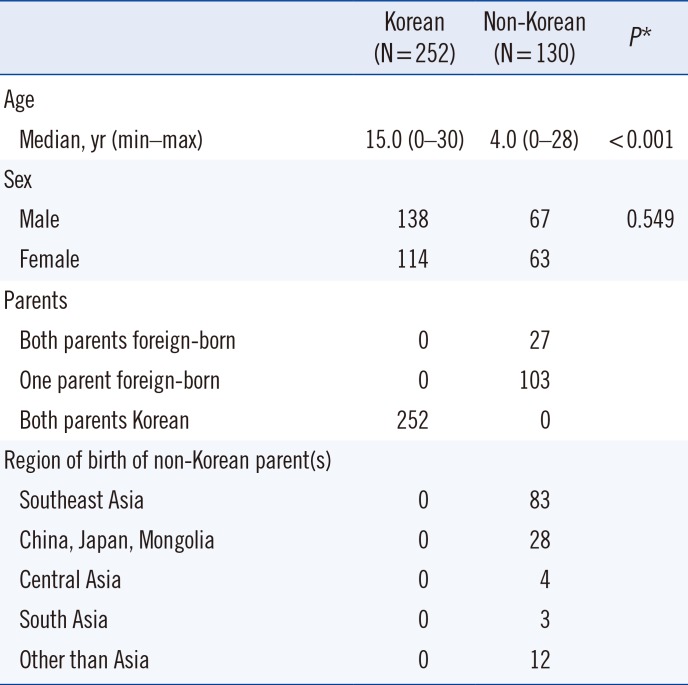
Table 2
Genotype frequencies by blood group in Korean and non-Korean subjects
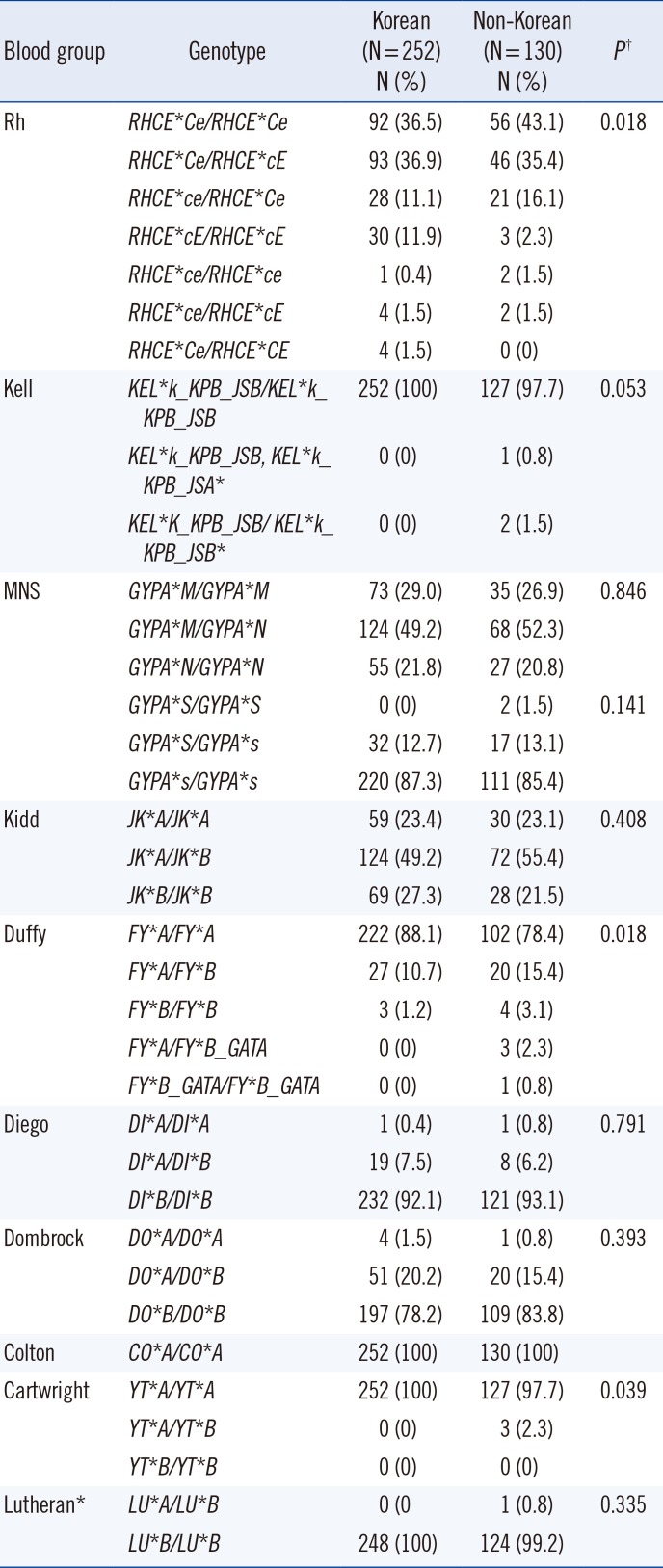
Table 3
Frequencies of predicted phenotypes by blood group in Korean (N=252) and non-Korean subjects (N=130)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download