Abstract
An inflammatory myofibroblastic tumor (IMT) is an uncommon, benign lesion characterized by the mesenchymal proliferation and infiltration of inflammatory cells composed primarily of lymphocytes and plasma cells. A percutaneous radiofrequency ablation (RFA) is an effective and safe therapeutic modality used for the management of liver malignancies. Here we report, for the first time, a case of IMT as a complication of RFA for hepatocellular carcinoma in a 61-year-old man with a Child's class A hepatitis B-related liver cirrhosis. Gastrohepatic fistula formation was pathologically proven and associated with the RFA. Such a longstanding inflammation of the fistula might have been a possible cause of the development of IMT in this case.
An inflammatory myofibroblastic tumor (IMT) is an uncommon, benign lesion which may simulate a malignancy on imaging studies. It is characterized by a mesenchymal proliferation featuring inflammatory cell infiltrates composed primarily of lymphocytes and plasma cells. Its pathogenesis is uncertain, although it is suggested that there are subsets of IMT with a neoplastic and reactive nature (1, 2).
A percutaneous radiofrequency ablation (RFA) is the most extensively used local ablation therapeutic modality for the management of primary or metastatic liver malignancies. The increasing appeal for this technique is attributed to its effectiveness, safety, and low morbidity rate (3). However, a number of potential major complications of an RFA have been described, occurring at a rate of 2%, including liver abscess, subcapsular hematoma, biliary stricture, perforation of the gastrointestinal tract and tumor seeding (4). Theoretically, an IMT could occur as a complication of an RFA because of thermal and mechanical injury caused by an RFA, which can induce an inflammatory change. However, until now, an IMT following an RFA has not been reported.
Herein, we report a case of an IMT involving a gastrohepatic ligament and adjacent liver and stomach, which developed after an RFA accompanied with a gastrohepatic fistula. To the best of our knowledge, this report is the first to present a case of an IMT in association with an RFA.
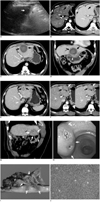
In a 61-year-old man with Child's class A hepatitis B-related liver cirrhosis, a 2.3 cm-sized mass was detected in Couinaud's segment III of the liver upon a routine screening US which was performed as a surveillance of the hepatocellular carcinoma (HCC) (Fig. 1A). On a subsequent contrast-enhanced CT, the mass showed a typical enhancement pattern consistent with HCC: subtle arterial enhancement and delayed washout (Fig. 1B). The serum α-fetoprotein (αFP) concentration at that time was 1.4 ng/ml. The mass was diagnosed as an HCC according to the 2005 guidelines of the American Association for the Study of Liver Diseases (5).
The patient underwent a percutaneous RFA to treat the tumor. The lesion was located peripherally and closely abutted against the stomach. Therefore, 500 ml of artificial ascites using dextrose 5% fluid was inserted between the left lateral hepatic segment and the stomach to prevent gastric thermal injury during the RFA. The tumor was ablated using the switching monopolar RFA technique (6). Three, 17-gauge, internally cooled electrodes with 3-cm active tips (Cool Tip Electrode; Valleylab, Bouler, CO) and a prototype multichannel RF generator (Taewong Medical Co., Koyang, Kyungki, Korea) to allow automatic switching of RF energy among three electrodes according to their impedance changes, were used to apply RF energy to the tumor. The lesion was ablated in one session and the total duration of ablation was 18 minutes at an average energy of 28,040 calories. The patient was asymptomatic after the procedure and was discharged the next day. Moreover, the patient was well and asymptomatic following a one month of routine outpatient follow-up.
The one-month follow-up CT images after the RFA (Fig. 1C, D) showed a thick-walled cystic change at the RFA site suggesting coagulation necrosis with liquefaction. The lesion closely abutted against the lesser curvature of the stomach at which asymmetric and heterogeneous wall thickening suggesting a thermal injury and subsequent chronic inflammation was shown (Fig. 1D). The patient did not complain of any symptoms and no laboratory tests was performed at that time.
The next routine follow-up CT at three months after the RFA showed a collapse of the previously seen, thick-walled, cystic lesion, and a newly appeared enhancing mass near the RFA site (Fig. 1E-G). On a coronal reformatted CT image obtained during the portal venous phase, the mass extended exophytically and the extrahepatic portion of the mass was in contact with the adjacent stomach and compressed it (Fig. 1G). Contrast-enhanced dynamic CT scans revealed that the mass enhanced slightly on both the arterial and portal venous phase CT images (Fig. 1F). Quantitatively, the attenuation of the mass was slightly higher (100 HU versus 82 HU) than that of the normal liver parenchyma on the arterial phase and with slightly lower (104 HU versus 117 HU) than that of the normal liver parenchyma on the portal venous phase. Transient hepatic attenuation differences around the lesion and infiltration around falciform ligament on arterial phase were also noted. At the time of interpretation, a tentative radiologic diagnosis was a marginal recurrence of HCC with suspicious gastric invasion.
The patient had no subjective symptom and there was no remarkable finding upon a physical examination. A laboratory examination showed a normal range of hemoglobin level (13.4 g/dL) and platelet count (150,000/µl), whereas a decreased WBC count (3,500/µl) with 55% segmental neutrophil and 30% lymphocyte was noted. In addition, mild peripheral eosinophilia (7%) was reported. Liver function tests were within normal limits and serum αFP levels showed no increase (1.5 ng/ml) from the previous test. A gastroscopy revealed a 2.5 cm submucosal mass at the lesser curvature side of the gastric angle. Small ulcerations were noted at the center of the lesion (Fig. 1H).
A tumorectomy of the liver and distal gastrectomy with Billroth II anastomosis were performed four months after performing the RFA. In the operative field, there was a 3×4 cm-sized mass involving segment III of the liver, which was adjacent to the stomach. The perioperative diagnosis also revealed a marginal recurrence of HCC with suspicious stomach invasion. A cut section of a gross specimen showed that the mass was primarily located in the perihepatic fatty tissue and extended to the adjacent hepatic parenchyma as well as to the proper muscle layer of the stomach. The area of coagulation necrosis induced by the previous RFA was pushed upwards by the mass (Fig. 1I). In addition, there were three openings of fistulous tract at the mucosa of the adherent stomach. The fistulous tract communicated with the liver parenchyma. A microscopic examination revealed relatively hypercellular, elongated rather than plump spindle cells (myofibroblasts) and a dense infiltration of inflammatory cells such as lymphocytes, plasma cells, and eosinophils (Fig. 1J). The final pathologic diagnosis was an IMT and was sub-categorized as having a compact spindle cell pattern.
The immunohistochemistry results showed positive results for CD35, CD3, leukocyte common antigen, L26, Ki67, while the antihepatocyte antigen, desmin, smooth muscle actin (SMA), anaplastic lymphoma kinase (ALK), CD34, CD21, and cytokeratin showed a negative result. The results were consistent with the diagnosis of an IMT.
A number of potential complications of an RFA have been described, occurring with a rate of almost 9% (4). Complications may be classified into three groups: vascular (i.e., portal vein thrombosis, hepatic vein thrombosis with partial hepatic congestion, hepatic infarction, and subcapsular hematoma), biliary (i.e., bile duct stenosis, biloma, abscess, and hemobilia), and extrahepatic (i.e., injury to the gastrointestinal tract, injury to the gallbladder, pneumothorax, hemothorax, and tumor seeding) (7). Theoretically, IMTs could occur as a complication of an RFA because thermal and mechanical injury caused by RFA can induce an inflammatory change which was considered to be one of the causes of developing an IMT. And as we describe here, IMTs can occur as a complication of an RFA.
Over the last two decades, IMTs have emerged from the broad category of inflammatory pseudotumors as a generic term applied to a variety of neoplastic and non-neoplastic entities that share a common histologic appearance; namely spindle cell proliferation with a prominent inflammatory infiltrate, with distinctive clinical, pathologic, and molecular features (8). Even though confusion remains regarding the distinction of these tumors from other lesions in the 'inflammatory pseudotumor' family, many pathologists recently started to reach a consensus that IMTs should be separately dealt with as a distinct disease entity because of the tendency for local recurrence. However, only a small risk of distant metastasis exists. Histologically, IMTs are characterized by a variably cellular spindle cell proliferation in a myxoid to collagenous stroma with a prominent inflammatory infiltrate composed primarily of plasma cells, and lymphocytes with occasional admixed eosinophils and neutrophils (8). Coffin et al. (9) described three basic histologic patterns, which are often seen in combination within the same tumor: a myxoid/vascular pattern, a compact spindle cell pattern, and a hypocellular fibromatosis-like pattern (9). The pathologic examination of our specimen showed was relatively hypercellular, with elongated rather than plump spindle cells (myofibroblasts) and a dense infiltration of inflammatory cells such as lymphocytes, plasma cells, and eosinophils. As a result the condition was sub-categorized as a compact spindle cell pattern.
This is the first case report in the literature describing an IMT extensively involving the liver, stomach, and perihepatic fatty tissue as a possible complication of an RFA. To find the reason why an IMT may have occurred following an RFA in our case, we should first consider what the unique features of our case were. The unique features might be an injection of artificial ascites and gastric wall injury with a subsequent gastrohepatic fistula. Theoretically, there might be a chance to injure the gastrohepatic ligament and perihepatic fatty tissue during the injection of artificial ascites. However, the direct association between the injection of ascites and injury could not be proven. Considering that gastrohepatic fistula can promote chronic and longstanding inflammation, which is one of the causes of a developing IMT, we can easily assume that the presence of a gastrohepatic fistula can be the possible cause of the occurrence of an IMT in our case. However, despite several reported cases on various kinds of fistulas following the RFA, an IMT is a very rare complication of an RFA. Indeed, even though there were three case reports describing gastrobiliary (n = 1) or enterobiliary (n = 2) fistula as a complication of an RFA, none of them developed an IMT (10, 11). Although we tried to minimize gastric injury during the RFA using an artificial ascites technique (12), we should confess to failing the prevention of gastric injury because artificial ascites were not able to remain at the nondependent portion of the body (i.e., hepatogastric recess), but was re-distributed into the dependent portion such as in the hepatorenal recess.
Imaging findings of IMTs are variable and usually nonspecific. The different ratios of cellular infiltration and hypocellular myxoid or fibrosis components observed pathologically in an IMT may explain the heterogeneity of the imaging findings. Because our case was classified as a pathologic subtype of a compact spindle cell pattern, we can guess that the imaging findings might resemble those of a gastrointestinal stromal tumor (GIST). Even though the dynamic enhancement pattern of a GIST has not yet been investigated, Kim et al. (13) insisted that most gastric GISTs show a good or moderate degree of homogeneous or heterogeneous enhancement on a portal phase CT, as in our case. However, given that this imaging finding is nonspecific and there is a previous history of HCC, reaching such a correct diagnosis for an IMT may be a difficult preoperative decision to make.
In a recent article by Lokken et al. (14), benign inflammatory nodules occurred in 2% of patients after a percutaneous ablation of renal tumors. Despite their rarity, the occurrence of benign inflammatory nodules are of clinical importance because they may mimic tumor seeding along the probe track. Even though the authors have described imaging features of these inflammatory nodules in detail, any differential points from tract seeding, aside from the interval decrease of the lesion size, could not be provided, which would prompt a biopsy or surgical excision for a correct diagnosis. Therefore, further investigation using a sufficient number of cases is strongly warranted in this regard.
In conclusion, an IMT can occur as a complication of an RFA when longstanding inflammation is present, possibly associated with gastrohepatic fistula formation as observed in this case. Therefore, radiologists should keep in mind that an IMT should be included as a differential diagnosis in patients with a growing mass and as a potential cause that could lead to longstanding inflammation following an RFA.
Figures and Tables
Fig. 1
61-year-old man with 2.3 cm-sized hepatocellular carcinoma in segment III of liver.
A. On sagittal US image, low echoic nodule (arrow) is seen in left lateral segment of liver. Note proximity of lesion to stomach (S).
B. Contrast-enhanced CT images display well-demarcated lesion (arrows) showing subtle enhancement on arterial phase (left) and washout of contrast material on portal venous phase (right).
C, D. Axial (C) and coronal reformatted (D) CT image obtained one month after radiofrequency ablation revealing thick-walled cyst-like appearance (arrows) of radiofrequency ablation site. Asymmetric and heterogeneously enhancing wall thickening (arrowheads in D) suggesting thermal injury is noted at adjacent lesser curvature of stomach.
61-year-old man with 2.3 cm-sized hepatocellular carcinoma in segment III of liver.
E-G. Follow-up portal phase CT image (E) obtained three months after radiofrequency ablation shows collapse (arrows) of previously seen, thick-walled, cystic lesion at radiofrequency ablation site. However, arterial (left in F) and portal (right in F) phase CT scans obtained 2.5 cm below (E) demonstrate ill-defined mass (arrows) with thick and irregular low attenuating rim. This lesion shows slight hyperattenuation on arterial phase (left in F) and slight low attenuation on portal venous (right in F) phase. Therefore, at time of interpretation, tentative radiologic diagnosis was marginal recurrence of hepatocellular carcinoma. Coronal reformatted CT image (G) obtained during portal venous phase displays mass (arrows) extending exophytically and to extrahepatic portion of mass, which was in contact with and compressing adjacent stomach (S). Gastroscopy (H) demonstrates 2.5 cm submucosal mass (arrows) at lesser curvature side of gastric angle. Two small ulcerations (*) observed at center of lesion.
61-year-old man with 2.3 cm-sized hepatocellular carcinoma in segment III of liver.
I. Cut section of gross specimen obtained after performing hepatic tumorectomy and distal gastrectomy shows that mass (arrows) was mainly located in perihepatic fatty tissue (F) and extended to adjacent hepatic parenchyma (L) as well as proper muscle layer (m) of stomach (S). Area of coagulation necrosis (dotted line) induced by previous radiofrequency ablation was pushed upwards by mass. In addition, there were three openings of fistulous tract at mucosa of adherent stomach. Moreover, fistulous tract communicated with liver parenchyma (not shown).
J. Upon microscopic examination (Hematoxylin & Eosin staining, ×100), elongated spindled myofibroblasts (*) were predominantly seen with heavy infiltration of lymphocytes and plasma cells. Inflammatory myofibroblastic tumor of compact spindle cell pattern was finally concluded as diagnosis.
References
1. Anthony PP, Telesinghe PU. Inflammatory pseudotumour of the liver. J Clin Pathol. 1986. 39:761–768.
2. Lawrence B, Perez-Atayde A, Hibbard MK, Rubin BP, Dal Cin P, Pinkus JL, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000. 157:377–384.
3. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.
4. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003. 23:123–134.
5. Bruix J, Sherman M. Practice Guidelines Committee. American Association for the Study of Liver Diseases. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005. 42:1208–1236.
6. Lee JM, Han JK, Kim HC, Choi YH, Kim SH, Choi JY, et al. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest Radiol. 2007. 42:163–171.
7. Akahane M, Koga H, Kato N, Yamada H, Uozumi K, Tateishi R, et al. Complications of percutaneous radiofrequency ablation for hepatocellular carcinoma: imaging spectrum and management. Radiographics. 2005. 25:S57–S68.
8. Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008. 61:428–437.
9. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995. 19:859–872.
10. Bessoud B, Doenz F, Qanadli SD, Nordback P, Schnyder P, Denys A. Enterobiliary fistula after radiofrequency ablation of liver metastases. J Vasc Interv Radiol. 2003. 14:1581–1584.
11. Falco A, Orlando D, Sciarra R, Sergiacomo L. A case of biliary gastric fistula following percutaneous radiofrequency thermal ablation of hepatocellular carcinoma. World J Gastroenterol. 2007. 13:804–805.
12. Kondo Y, Yoshida H, Shiina S, Tateishi R, Teratani T, Omata M. Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg. 2006. 93:1277–1282.
13. Kim HC, Lee JM, Kim KW, Park SH, Kim SH, Lee JY, et al. Gastrointestinal stromal tumors of the stomach: CT findings and prediction of malignancy. AJR Am J Roentgenol. 2004. 183:893–898.
14. Lokken RP, Gervais DA, Arellano RS, Tuncali K, Morrison PR, Tatli S, et al. Inflammatory nodules mimic applicator track seeding after percutaneous ablation of renal tumors. AJR Am J Roentgenol. 2007. 189:845–848.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download