Abstract
Adenosquamous carcinomas of the duodenum are extremely rare neoplasms in which both glandular and squamous elements demonstrate malignant characteristics. Few cases of adenosquamous carcinoma involving the second or third segment of the duodenum have been reported in the literature. Herein, we report the first case of adenosquamous carcinoma of the bulb of the duodenum that mimicked subepithelial tumor on computed tomography in a 59-year-old man.
Malignant neoplasms arising from the small bowel are relatively rare, accounting for only 1–2% of all gastrointestinal (GI) cancers (12). Among several subtypes of these small intestinal cancers, adenosquamous carcinomas (ASCs) are rare tumors of the bowel containing both malignant glandular and squamous components (1). ASCs have been mostly reported in the gastroesophageal junction and the anal canal where glandular and squamous epithelium are normally juxtaposed (3). They have also been reported in other parts of the GI tract as esophagus, stomach and colon (1). To date, a very few cases of small intestinal ASCs have been reported in the literature and only 10 cases of them were duodenal ASCs involving second or third segment of duodenum (1245678). Herein, we report another case of duodenal ASC that mimick subepithelial tumor on computed tomography (CT), and to the best of our knowledge, this is the first reported case of duodenal ASC arising from duodenal bulb.
A 59-year-old male presented to emergency department of our hospital with history of melena. The general physical and systemic examination was unremarkable. The laboratory examination revealed a hemoglobin level of 7.9 g/dL, hematocrit level of 24.8%. All tumor marker levels were normal. Upper GI endoscopy revealed a subepithelial mass at duodenal bulb and spurting bleeding on the surface of the mass (Fig. 1A), and epinephrine injection was done for hemostasis. For further evaluation of the mass, CT with contrast enhancement was performed. On CT (Fig. 1B), a 3 cm sized relatively well-circumscribed hypoattenuating mass was noted at duodenal bulb, and the mass was surrounded by hyperattenuating duodenal wall, so it presented the appearance of subepithelial tumor. The mass showed endoluminal growth pattern resulting severe gastric distension, suggesting gastric outlet obstruction. There was no evidence of active bleeding on CT. And also, there was no invasion into adjacent organs and no evidence of distant metastatic lesion within covered range. The major vessels were free of this tumor. This patient underwent duodenal segmental resection with gastrojejunostomy, and grossly there was a well-defined 4 cm sized subepithelial solid mass with whitish yellow granular cut surface, involving entire duodenal wall (Fig. 1C–F). The duodenal mucosa was only focally eroded. Microscopically, the tumor was composed of biphasic components, including moderately differentiated adenocarcinoma and poorly differentiated squamous cell carcinoma. The two components were intimately mixed together without a clear border. Adenocarcinoma showed well-developed luminal spaces, mucin droplets, and partly micropapillary pattern. Squamous cell carcinoma was arranged in nests of polygonal cells with intercellular bridges. On immunohistochemical stains, the tumor cells are diffusely positive for cytokerain 19. The glandular part was immunoreactive for cytokeratin 7 whereas squamous part was positive for cytokeratin 5/6 and p40. Accordingly, it was diagnosed with ASC of duodenal bulb, with serosal involvement. The patient is doing fine at 3 months follow up.
The small intestine is the largest part of GI tract, encompassing nearly 90% of its mucosal surface. Interestingly, it only contributes minimally to the total tumor burden from the GI tract, only 1–2% of GI malignancies originate from the small intestine (12). The ileum carries the majority of the small intestinal tumor burden, followed by the duodenum, and lastly the jejunum (9). The majority of duodenal cancers originate from the descending duodenal segment, followed by the horizontal and ascending segments, and rarely from the proximal horizontal segment called duodenal bulb (10).
Among more than 40 histological subtypes of small intestinal malignancies that have been described in the literature, ASCs are extremely rare, so ASCs have been reported as isolated case reports (1245678). In the majority of these cases, the tumor originates from the descending segment (2nd portion) of duodenum. Clinical presentations of patients with duodenal ASC are variable and include symptoms like abdominal pain, anemia, nausea, vomiting, and melena due to GI bleeding (2). And duodenal ASCs are irrespective of sex or age (2).
Radiologic findings of reported cases of duodenal ASCs are also variable depending upon the area of origin (1). It ranges from duodenal caliber change with minimal adjacent fat stranding (2) to discrete soft tissue mass with dilatation of common bile duct and pancreatic duct (7). In our case, unusually, ASC appeared as well-circumscribed mass that mimicked subepithelial tumor on CT. Therefore, at first, we considered possibilities of GI stromal tumor or adenocarcinoma with unusual radiologic feature as a differential diagnosis. And, because the ASC was located at duodenal bulb, it caused gastric outlet obstruction instead of pancreaticobiliary duct obstruction.
Diagnosis of duodenal ASC can be established with upper GI endoscopy with biopsy and contrast enhanced CT of abdomen (1). However, preoperative diagnosis of duodenal ASC is difficult because of the lack of defining characteristics in imaging studies and the difficulty in acquiring both malignant glandular and squamous components by limited biopsy (5). In the preoperative biopsy of the presented case, there was only benign duodenal epithelium with gastric metaplasia, hyperplastic change and chronic inflammation in the biopsy specimen. The limited biopsy may have been due to a characteristic subepithelial growth pattern of the tumor.
The treatment of choice is the surgical resection. Due to limited number of these cases, there is no effective neoadjuvant or adjuvant therapy documented in the literature (1). Most of reported duodenal ASCs showed poor clinical outcomes (2). Most patients experienced early distant metastasis and short survival after surgery (5). In our case, there were no evidence of local recurrence or distant metastasis on CT and positron emission tomography- CT and no abnormal laboratory findings at 3 months follow up after the surgery, but it is hard to tell the prognosis of this patient because term of follow up is not long enough yet. The true prognosis of ASC in duodenum remains to be investigated after more number of cases are reported.
In summary, we present the first case of ASC in duodenal bulb that mimicked subepithelial tumor on CT. On account of the rareness of the ASC of the duodenum, very limited information exists in the medical literature regarding the radiologic findings, clinical features and ideal management strategies. Further cases of these uncommon cancers need to be identified and reported for better understanding of these tumors. And even if a mass in duodenum appears to be a subepithelial tumor on imaging studies, radiologists should consider possibility of ASC, though it is exceedingly rare disease entity.
Figures and Tables
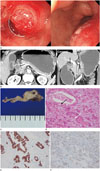 | Fig. 1A 59-year-old man with adenosquamous carcinoma involving duodenal bulb.
A. Upper gastrointestinal endoscopy reveals a subepithelial mass at duodenal bulb and spurting bleeding on the surface of the mass (left), and epinephrine injection is done for hemostasis (right).
B. Axial and coronal images of contrast enhanced CT scan show a 3 cm sized well-circumscribed hypoattenuating mass surrounded by hyperattenuating duodenal wall at the bulb of duodenum (arrows). The lesion causes gastric outlet obstruction.
C. Grossly, a well-defined solid mass shows granular, necrotic, and hemorrhagic cut surface involves the duodenal wall.
D. Microscopically, the lesion displays glands with intracellular mucin (black arrow) and islands with intercellular bridges and keratin pearl typical of squamous differentiation (white arrow) (hematoxylin and eosin stain, × 100).
E. Immunohistochemically, the glandular part is immunoreactive for cytokeratin 7 (× 200).
F. The squamous part is positive for p40 (× 200).
|
References
1. Daga G, Kerkar P. Adenosquamous carcinoma of the duodenum: a rare entity. Indian J Surg Oncol. 2016; 7:470–474.

2. Hammami MB, Chhaparia A, Piao J, Zhou Y, Hachem C, Lai J. Mixed adenocarcinoma and squamous cell carcinoma of duodenum: a case report and review of the literature. Case Rep Gastroenterol. 2017; 11:402–410.

3. de la Cruz A, de la Cruz E, Sanchez MJ, Ortiz S, Lobato A, Merino E. Adenosquamous carcinoma of the duodenum. an immunohistochemical study. Pathol Res Pract. 1993; 189:481–485. discussion 485-487.
4. Takayoshi K, Ariyama H, Tamura S, Yoda S, Arita T, Yamaguchi T, et al. Intraluminal superior vena cava metastasis from adenosquamous carcinoma of the duodenum: a case report. Oncol Lett. 2016; 11:605–609.

5. Yang SJ, Ooyang CH, Wang SY, Liu YY, Kuo IM, Liao CH, et al. Adenosquamous carcinoma of the ampulla of Vater - a rare disease at unusual location. World J Surg Oncol. 2013; 11:124.

6. Ueno N, Sano T, Kanamaru T, Tanaka K, Nishihara T, Idei Y, et al. Adenosquamous cell carcinoma arising from the papilla major. Oncol Rep. 2002; 9:317–320.

7. Kshirsagar AY, Nangare NR, Vekariya MA, Gupta V, Pednekar AS, Wader JV, et al. Primary adenosquamous carcinoma of ampulla of Vater-a rare case report. Int J Surg Case Rep. 2014; 5:393–395.

8. Hoshimoto S, Aiura K, Shito M, Kakefuda T, Sugiura H. Adenosquamous carcinoma of the ampulla of Vater: a case report and literature review. World J Surg Oncol. 2015; 13:287.

9. Hatzaras I, Palesty JA, Abir F, Sullivan P, Kozol RA, Dudrick SJ, et al. Small-bowel tumors: epidemiologic and clinical characteristics of 1260 cases from the connecticut tumor registry. Arch Surg. 2007; 142:229–235.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download